e-ISSN: 2319-9849
e-ISSN: 2319-9849
Department of Chemistry, Dr.Hari Singh Gour University, A Central University, Sagar - 470003, Madhya Pradesh, India
Received date: 12/06/2013; Revised date: 22/06/2013; Accepted date: 29/06/2013
Visit for more related articles at Research & Reviews: Journal of Chemistry
Nitrogen containing compounds find wide application as structural components in pharmaceuticals and agrochemicals due to their marked biological activities. Among biologically active heterocycles, Indole and its derivatives enjoys wide range of therapeutic and pharmacological properties as anti convulsant, analgesic, sedative anti-depressive,anti-carcinogenic and hypnotic agents. In this study a new series of Novel 3-(3,5-dimethyl-4H-pyrazol-4-ylidene)-1-[(4-methyl phenyl)sulphonyl]-1,3-dihydro-2H-indole-2-one (4) was synthesized. Structure of the title compound was characterized by spectral techniques like FT-IR and 1H NMR.
double salt, aceto -nitrile, para-toluene sulphonyl chloride, pyrazole moiety.
The indole framework is a medicinally relevant scaffold and has become widely identified as a privileged structure. The indole nucleus is present in thousands of issolated natural products with diverse therapeutic activities, such as antiviral activities, antifungal activities, antimicrobial activities, antitumour activities and and recently antituberculosis activities. So to met the emerging demands, many Laboratories and Industrial units are seeking new Anti –mycobacterial agents that could confer greater selectivity and lower toxicity [1-3]. Continuing our research for Anti-tubercular agents we have synthssized some derivatives of 3-(3,5-dimethyl-4H-pyrazol-4-ylidene)-1-[(4-methyl phenyl)sulphonyl]-1,3-dihydro-2H-indole-2-one (4a-d). These compounds were characterised by their spectral analysis(FT-IR, HI-NMR ,MS).
Drugs and Chemicals
Reactants for the synthesis of molecule 4 were procured from Sigma aldrich ltd. Media and other materials for antimicrobial activity were procured from Hi-media ltd. All the drugs and chemicals except the test compound (IVa) were dissolved or diluted in distilled water and used for the experimentation purpose. 4a-d was dissolved in 100% DMSO and dilutions were made with distilled water so that the final concentration of DMSO did not exceed (0.1 % v/v).
Experimental
Melting points were determined using open capillary tube in Toshniwal Melting point apparatus and are presented without any correction. The infrared (IR) spectra were recorded on a FTIR-8310 Shimadzu spectrometer using potassium bromide pellets. The proton nuclear magnetic resonance (1H-NMR) specta were recorded on AMX 400 at 200 MHz using tetramethylsilane (TMS) as the internal standard and DMSO as solvent, collected from Central Instrumental Lab. Chandigarh University. All reagents were of the highest purity commercially available. The chemical shifts are expressed inpart per million (ppm) downfield from the internal standard; the coupling constants are in Hz, and signals are quoted ass(singlet), d(doublet), t (triplet), q (quartet), or m (multiplet). The purity of the compounds was checked by Thin Layer Chromatography using .Merck Pre-coated silica gel GF aluminium plates and Ethyl acetate : Chloroform (15:85) as solvent system.
Synthesis of Potassium Aluminium Sulphate Dodecahydrate
Add 25 ml of 3M KOH in a 250 ml beaker containingAluminium pieces. Proceed the reaction hood and filter it while hot to remove undissolved carbon particles. Cool the reaction mixture and acidify it with continuous stirring using 3M H2SO4. Concentrate the mixture and allow it to stand for overnight to crystallize Potassium aluminium sulphate dodecahydrate, a catalyst(Double Salt) [2-4].
Synthesis of 3-(2-oxo-1,2-dihydro-3H-Indole-3-ylidene) pentane-2,4-dione(1a)
Took 0.05mole of substituted isatin and o.05 mole of acetyl-acetone in a conical flask. Mixture was subjected to cool in an ice bath, followed by addition of 1ml piperidine with continuous stirring. The reaction mixture was kept at freezing point temperaturefor 3 hours followed by addition of cold ethanol to break the lumps, filter the product and wash it with ethanol cold, dried vaccum conditions.Mp:129 0C. Yield: 71%. 1HNMR(200 MHz,TMS):δ 7.4(s, 3H, HAr) ,4.1(s, 1H, NH) 2.3(s, 3H, MeH), IR(KBr) Cm-1 :1453 Cm-1(C-C st), 1571Cm-1(C=C) ,2943 Cm-1, (C-H), 1705 Cm-1(C=O), 3267 Cm-1(N-H); MS: m/z 228 (M+, I= 67% ), m/z 43 (I= 100%).
Synthesis of 5-Chloro- 3-(2-oxo-1,2-dihydro-3H-Indole-3-ylidene) pentane-2,4-dione(1b)
Mp:120 °C. Yield: 73%. 1HNMR(200 MHz,TMS):δ 7.4(s, 3H, HAr) ,4.1(s, 1H, NH) 2.3(s, 3H, MeH), IR(KBr) Cm-1 :1453 Cm-1(C-C st), 1571Cm-1(C=C) ,803 Cm-1 (C-Cl), 1705 Cm-1(C=O), 3267 Cm-1(N-H); MS: m/z 228 (M+, I= 67% ), m/z 43 (I= 100%).
Synthesis of 5-Bromo- 3-(2-oxo-1,2-dihydro-3H-Indole-3-ylidene) pentane-2,4-dione(1c)
Mp:133°C. Yield: 67%. 1HNMR(200 MHz,TMS):δ 7.4(s, 3H, HAr) ,4.1(s, 1H, NH) 2.3(s, 3H, MeH), IR(KBr) Cm-1 :1453 Cm-1(C-C st), 1571Cm-1(C=C) ,713 Cm-1 (C-Br), 1705 Cm-1(C=O), 3267 Cm-1(N-H); MS: m/z 228 (M+, I= 67% ), m/z 43 (I= 100%).
Synthesis of 5-Nitro- 3-(2-oxo-1,2-dihydro-3H-Indole-3-ylidene) pentane-2,4-dione(1d)
Mp:109°C. Yield: 63%. 1HNMR(200 MHz,TMS):δ 7.4(s, 3H, HAr) ,4.1(s, 1H, NH) 2.3(s, 3H, MeH), IR(KBr) Cm-1 :1453 Cm-1(C-C st), 1571Cm-1(C=C) ,721 Cm-1 (C-NO2), 1705 Cm-1(C=O), 3267 Cm-1(N-H); MS: m/z 228 (M+, I= 67% ), m/z 43 (I= 100%).
Synthesis of 3-(3,5-dimethyl-4H-pyrazol-4-ylidene)-1,3-dihydro-2H-Indol-2-one.(2a) [5]
0.05 moles of 1a-d and 0.06 moles of hydrazine were added in 40 ml of ethanol containing 500 mg of double salt with continuous stirring for 2 hours,followed by dilution with water. Extract the product with ethylacetate successively for three times. Decant it and passed it through sodium sulphate.Kept it under dessicator for drying.
Mp:176 °C. Yield: 78%. 1HNMR(200 MHz,TMS):δ 7.4(s, 3H, HAr) ,4.1(s, 1H, NH) 2.3(s, 3H, MeH), IR(KBr) Cm-1 :1453 Cm-1(C-C st), 1571Cm-1(C=C) ,2949 Cm-1 (C-H), 1709 Cm-1(C=O), 3267 Cm-1(N-H.), 1207 Cm-1(C=N),1310 Cm-1(N-N); MS: m/z 224 (M+, I= 61% ), m/z 15 (I= 100%).
Synthesis of 5-Chloro- 3-(3,5-dimethyl-4H-pyrazol-4-ylidene)-1,3-dihydro-2H-Indol-2-one.(2b)
Mp:183 °C. Yield: 58%. 1HNMR(200 MHz,TMS):δ 7.4(s, 3H, HAr) ,4.1(s, 1H, NH) 2.3(s, 3H, MeH), IR(KBr) Cm-1 :1453 Cm-1(C-C st), 1571Cm-1(C=C) ,803 Cm-1 (C-Cl), 1705 Cm-1(C=O), 3267 Cm-1(N-H) 1207 Cm-1(C=N),1310 Cm-1(N-N); MS: m/z 224 (M+, I= 61% ), m/z 15 (I= 100%).
Synthesis of 5-Bromo- 3-(3,5-dimethyl-4H-pyrazol-4-ylidene)-1,3-dihydro-2H-Indol-2-one.(2c)
Mp:207°C. Yield: 69%. 1HNMR(200 MHz,TMS):δ 7.4(s, 3H, HAr) ,4.1(s, 1H, NH) 2.3(s, 3H, MeH), IR(KBr) Cm-1 :1453 Cm-1(C-C st), 1571Cm-1(C=C) ,713 Cm-1 (C-Br), 1705 Cm-1(C=O), 3267 Cm-1(N-H),1207 Cm-1(C=N),1310 Cm-1(N-N); MS: m/z 224 (M+, I= 61% ), m/z 15 (I= 100%).
Synthesis of 5-Nitro- 3-(3,5-dimethyl-4H-pyrazol-4-ylidene)-1,3-dihydro-2H-Indol-2-one.(2d)
Mp:167°C. Yield: 61%. 1HNMR(200 MHz,TMS):δ 7.4(s, 3H, HAr) ,4.1(s, 1H, NH) 2.3(s, 3H, MeH), IR(KBr) Cm-1 :1453 Cm-1(C-C st), 1571Cm-1(C=C) ,721 Cm-1 (C-NO2), 1705 Cm-1(C=O), 3267 Cm-1(N-H) 1207 Cm-1(C=N), 1310 Cm-1(N-N) MS: m/z 224 (M+, I= 61% ), m/z 15 (I= 100%).
Synthesis of 3-(3,5-dimethyl-4H-pyrazol-4-ylidene)-1-ethyl-1,3-dihydro-2H-Indol-2-one.(3a)
A suspension of 2a-d(1.96 g, 6.0 mmol), 1.5 g k2CO3 and 0.97 ml methyl iodide(12mmol) in 30 ml acetonitrile was stirred at 68C for 2h.After the reaction mixture was cooled and filtered, the transparent yellow filtrate was evaporated in vaccum and then brown residue was recrystallized from ethyl acetate,obtaining the 3a-j as a pale coloured solid. .Mp:209 0C. Yield: 73%. 1HNMR(200 MHz,TMS):δ 7.4(s, 3H, HAr) ,4.1(s, 1H, NH) 2.3(s, 3H, MeH),i.9(t, 3H,MeH). IR(KBr) Cm-1 :1453 Cm-1(C-C st), 1571Cm-1(C=C) ,2949 Cm-1 (C-H), 1709 Cm-1(C=O), 3267 Cm-1(N-H.), 1207 Cm-1(C=N), 1310 Cm-1(N-N),983 Cm-1(C-N); MS: m/z 252 (M+, I= 71% ), m/z 29 (I= 100%).
Synthesis of 5-Chloro- 3-(3,5-dimethyl-4H-pyrazol-4-ylidene)-1-ethyl-1,3-dihydro-2H-Indol-2-one.(3b) [6,7].
Mp:223°C.Yield: 64%. 1HNMR(200MHz,TMS):δ 7.4(s, 3H, HAr) ,4.1(s, 1H, NH) 2.3(s, 3H, MeH),i.9(t, 3H,MeH). IR(KBr) Cm-1 :1453 Cm-1(C-C st), 1571Cm-1(C=C) ,717 Cm-1 (C-Br),1709 Cm-1(C=O), 3267 Cm-1(N-H.), 1207 Cm-1(C=N), 1310 Cm-1(N-N),983 Cm-1 (C-N); MS: m/z 252(M+, I= 71% ), m/z 29 (I= 100%).
Synthesis of 5-Nitro- 3-(3,5-dimethyl-4H-pyrazol-4-ylidene)-1-ethyl-1,3-dihydro-2H-Indol-2-one.(3d)
Mp:I83°C.Yield: 61%. 1HNMR(200MHz,TMS):δ 7.4(s, 3H, HAr) ,4.1(s, 1H, NH) 2.3(s, 3H, MeH),i.9(t, 3H,MeH). IR(KBr) Cm-1 :1453 Cm-1(C-C st), 1571Cm-1(C=C) ,713 Cm-1 (C-NO2), 1709 Cm-1(C=O), 3267 Cm-1(N-H.), 1207 Cm-1(C=N), 1310 Cm-1(N-N),983 Cm-1(C-N); MS: m/z 252(M+, I= 71% ), m/z 29 (I= 100%).
Synthesis of 3-(3,5-dimethyl-4H- pyrazol-4-ylidene)-1-[(4-methyl phenyl) sulphonyl]-i,3-dihydro-2H-indol-2-one (4a) [8].
0.08 moles of 3 was N- alkylated with 1.7 ml para-toluene-sulphonyl chloride in 49 ml acetonitrile in presence of 0.68g NaH. The reaction mixture was heated upto 80C with constant stirring for about 6-8 hrs. After that reaction mixture was cooled and filtered, acidified using dilute HCl. The product was filtered out and dried in vaccum conditions. .Mp:2890C.Yield: 73%. 1HNMR(200MHz,TMS):δ 7.4(s, 3H, HAr) ,4.1(s, 1H, NH) 2.3(s, 3H, MeH), IR(KBr) Cm-1 :1453 Cm-1(C-C st), 1571Cm-1(C=C) ,2949 Cm-1 (C-H), 1709 Cm-1(C=O), 3267 Cm-1(N-H.), 1207 Cm-1(C=N),1310 Cm-1(N-N),983 Cm-1(C-N), 1049Cm-1(S-N), 1025 Cm-1(C-S); MS: m/z 378(M+, I= 69% ), m/z 91 (I= 99%).
Synthesis of 5-Chloro- 3-(3,5-dimethyl-4H- pyrazol-4-ylidene)-1-[(4-methyl phenyl) sulphonyl]-i,3-dihydro-2H-indol-2-one (4b)
Mp:328 °C. Yield: 59%. 1HNMR(200 MHz,TMS):δ 7.4(s, 3H, HAr) ,4.1(s, 1H, NH) 2.3(s, 3H, MeH),. IR(KBr) Cm-1 :1453 Cm-1(C-C st), 1571Cm-1(C=C) ,797 Cm-1 (C-Cl), 1709 Cm-1(C=O), 3267 Cm-1(N-H.), 1207 Cm-1(C=N), 1310 Cm-1(N-N),983 Cm-1(C-N). 1049Cm-1(S-N), 1025 Cm-1(C-S); MS: m/z 378 (M+, I= 69% ), m/z 91 (I= 99%).
Synthesis of 5-Bromo- 3-(3,5-dimethyl-4H- pyrazol-4-ylidene)-1-[(4-methyl phenyl) sulphonyl]-i,3-dihydro-2H-indol-2-one(4c)
Mp:289°C.Yield: 58%. 1HNMR(200MHz,TMS):δ 7.4(s, 3H, HAr) ,4.1(s, 1H, NH) 2.3(s, 3H, MeH),i.9(t, 3H,MeH). IR(KBr) Cm-1 :1453 Cm-1(C-C st), 1571Cm-1(C=C) ,717 Cm-1 (C-Br), 1709 Cm-1(C=O), 3267 Cm-1(N-H.), 1207 Cm-1(C=N), 1310Cm-1(N-N),983 Cm-1(C-N) 1049Cm-1(S-N), 1025 Cm-1(C-S); MS: m/z 378 (M+, I= 69% ), m/z 91 (I= 99%).
Synthesis of 5- Nitro-3-(3,5-dimethyl-4H-pyrazol-4-ylidene)-1-[(4-methyl phenyl) sulphonyl]-1,3-dihydro-2H-Indol-2-one(4d)
Mp:3050C.Yield: 68%. 1HNMR(200MHz,TMS):δ 7.4(s, 3H, HAr) ,4.1(s, 1H, NH) 2.3(s, 3H, MeH), IR(KBr) Cm-1 :1453 Cm-1(C-C st), 1571Cm-1(C=C) ,713 Cm-1 (C-NO2), 1709 Cm-1(C=O), 3267 Cm-1(N-H.), 1207 Cm-1(C=N), 1310 Cm-1(N-N),983 Cm-1(CN) 1049Cm-1(S-N), 1025 Cm-1(C-S); MS: m/z 378 (M+, I= 69% ), m/z 91 (I= 99%).
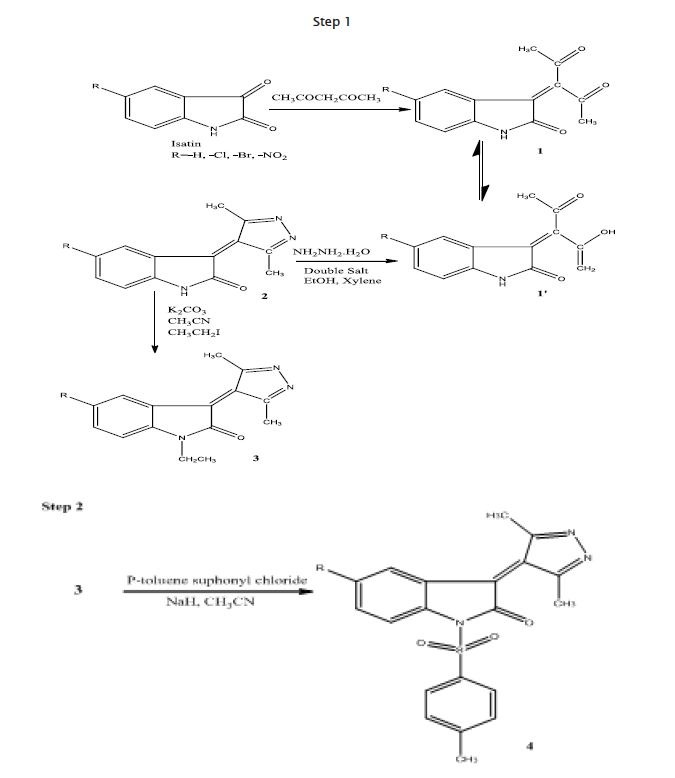
The synthetic pathway followed in the prepration of the compounds is outlined in the scheme.
Pentane-2,4-dione(1) was obtained by reacting 5-substituted Isatin with acetyl acetone in presence of piperidine.It undergoes tautomerizes to(1’). Compound(1) was treated treated with Hydrazine in presence of Double Salt acting as catalyst with continuous stirring for 2-3 hrs to afford 3-(3,5dimethy4H-pyrazol-4-ylidene)-1,3-dihydro-2H-Indol-2-one.(2)Compound(2)was N-alkylated with ethyl iodide in acetonitrile to give 3-(3,5-dimethyl-4H-pyrazol-4-ylidene)-1-ethyl-1,3-dihydro-2H-Indol-2one.(3).Compound(3) was further treated with p-toluene- sulphonyl chloride in acetonitrile in presence of NaH to give 3-(3,5-dimethyl-4H- pyrazol-4-ylidene)-1-[(4-methyl phenyl) sulphonyl]-i,3-dihydro-2H-indol-2-one (4). The IR spectrum of compound (1) displayed strong bands at 3267 cm-1 and 1705 cm-1 respectively due to N-H and C=O stretching.The C-H stretching in compound (1) was observed at 3025 cm-1 while in case of compound (2), a strong peak was observed at 2910cm-1 due to C-CH3 stretching supporting the formation of 2.A peak wasobserved at 3543 cm-1 because of O-H stretching supporting tautomerization.Peak is observed at 1310 due to N-N stretching.In addition two intense peaks were observed at 1049 Cm-1 and 1025 Cm-1 due to respective S-N and C-S Stretchings.In the 1HNMR of 1-3 a singlet due to NH is observed at 7.4. Also a singlet is observed at 2.3 due to 3H of Benzene ring(HAr). At 7.3-7.5 a singlet is observed due to 3H of aromatic ring.
So we have concluded that Double Salt i.e KAl(SO4)2.12H2O can ba a better catalyst because it is non-toxic, inexpensive, re-usable and easily available for the synthesis of 3-(3,5-dimethyl-4H- pyrazol-4-ylidene)-1-[(4-methyl phenyl) sulphonyl]-1,3-dihydro-2H-indol-2-one (4) . Also derivatives of 3-(3,5-dimethyl-4H- pyrazol-4-ylidene)-1-[(4-methyl phenyl) sulphonyl]-i,3-dihydro-2H-indol-2-one (4) possessing pyrazole moiety have also gained widespread intrest due to their respective broad potential anti-mycobacteriall activity and anticancer activities.
https://transplanthair.istanbul
https://hairclinicturkey.co
https://hairclinicistanbul.co
https://besthairtransplant.co
https://hairtransplantistanbul.co