E- ISSN: 2320 - 3528
P- ISSN: 2347 - 2286
E- ISSN: 2320 - 3528
P- ISSN: 2347 - 2286
Mushtaq M1, Sultana B1, Akram S1, Adnan A1, Owusu-Apenten R2 and Nigam Singh P2*
1Department of Chemistry, University of Agriculture, Faisalabad-38040, Pakistan
2School of Biomedical Sciences, Ulster University, Coleraine, BT52 1SA, UK
Received date: 02/05/2016; Accepted date: 26/05/2016; Published date: 06/06/2016
Visit for more related articles at Research & Reviews: Journal of Microbiology and Biotechnology
Objective: This study examines enzyme-assisted recovery of polyphenols from pomegranate peel using a multi-response optimization process. Methods: Enzyme pre-treatment factors (enzyme dose, incubation time, temperature and pH) were optimized for extract yield, total phenol concentration (TPC), radical scavenging capacity (RSC) and trolox equivalent antioxidant capacity (TEAC). Results: Pre-treatment of pomegranate peel with 3.8% of cocktail enzyme at 6.7 pH and 41ºC for 85 min produced a mass yield of 65.89 ± 2.64 g/100 g of crude extracts (3 fold increase compared to conventional solvent extraction) with a total phenols concentration of 277.93 ± 6.17 mg GAE/g dry weight, 398.70 ± 3.06 μmol trolox equivalence /g and 73.15 ± 0.69 μg/mL radical quenching capacity (IC50). Conclusion: Enzyme pre-treatment improved the efficiency of extraction of polyphenols from pomegranate peel.
Pomegranate peel, Polyphenols, Extraction, Antioxidants, Enzyme pretreatment.
Pomegranate (Punica granatum) is an important fruit for direct consumption and for juice extraction. The peel of pomegranate (pomegranate peel) constitutes more than 40% of the fruit and is a valuable source of antioxidants [1]. Polyphenols from pomegranate are associated with a number of health benefits including a role as, antioxidant, antimicrobial agent, antiinflammatory agent, anti-proliferative agent, lipase inhibitor, and inhibitor for α-glycosidase [2-4]. Indeed, pomegranate peel antioxidant is being examined as natural ingredients for food processing and preservation [3].
Attempts to improve the extractability of polyphenol antioxidants from pomegranate peel have been described recently involving various solvents [5,6], ultrasound assisted extraction [7-10] or microwave assisted extraction [11]. Optimization investigations showed that the extraction kinetics and efficiency could be affected by the solvent choice, extraction time, temperature, peel particle size [8,10,12-15].
In previous work, the current team successfully optimized enzyme-assisted extraction of polyphenols from sweet-lime [16], watermelon [17] and also examined super-fluid extraction of pomegranate peel [18]. Currently, the effect of enzymatic pre-digestion on the extractability of polyphenols from pomegranate peel has not been reported. The aims of the research presented in this paper were to evaluate enzyme-assisted solvent extraction (EASE) of polyphenols from pomegranate peel and to characterize the major components by HPLC, and the degree of antioxidant activity. The outcomes evaluated include, the extract yield, total phenolic concentration (TPC), radical scavenging capacity (RSC) and Trolox Equivalent antioxidant capacity (TEAC).
Materials and chemicals
Peels of pomegranate “Tarnab Gulabi” ecotype were collected from a local juice processor in Pakistan, rinsed with distilled water, and dried under ambient conditions (35ºC). Samples were processed into homogenous powder of 0.5 mm diameter and packed into airtight bags. The enzyme formulations used contained cellulases, pectinases and proteases as described previously [18]. Reagents including, 2,2-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid) di-ammonium salt (ABTS), 2,2-diphenyl-1-(2,4,6- trinitrophenyl) hydrazyl radical (DPPH), 6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (Trolox), Folin-Ciocalteu reagent (FCR), and 2,6-di-tert-butyl-4-methylphenol (BHT) and phenolic acids including 3, 4-dihydroxy benzoic, p-hydroxy benzoic, gallic, linoleic, vanillic, caffeic, p-coumaric, ferulic, syringic and sinapic acids were purchased from Sigma, St. Louis (USA). Ammonium thiocyanate, potassium persulfate, potassium ferrocyanide, sodium carbonate, and acetic acid were supplied by Merck (Darmstadt, Germany). Ultra-pure deionized water was from Milli-Q Plus system (Millipore, Bedford, MA, USA) whereas 96-well microplates used were of Fisher Scientific (Pittsburgh, PA, USA).
Experimental design
Preliminary screening experiments were performed to establish enzyme concentration (EC), temperature (T), incubation time (t) and pH which affected pomegranate peel breakdown and liberation of polyphenol. The factors were further investigated at axial (eight runs) and factorial (eight runs) levels (-α, -1, +1, +α) in rotatable central composite design (α=1.82) as expressed in Table 1. Five replicate runs at center points (α=0) were made to estimate main effects and error. Responses including extract yield (yield), total phenols concentration (TPC), trolox equivalent antioxidant capacity (TEAC) and DPPH radical scavenging capacity were modeled using a 2nd order polynomial equation using Statistical Software Design Expert (version 10).
| Run | Pre-treatment conditions | Response measured | ||||||
|---|---|---|---|---|---|---|---|---|
| EC (%) (A) |
T (°C) (B) |
t (Min) (C) |
pH (D) |
Extract yieldK | TPCL | TEACM | DPPHN | |
| 1 | 44 (1) | 54 (1) | 100 (1) | 56 (-1) | 35 | 143 | 30 | 47 |
| 2 | 35 (0) | 45 (0) | 75 (0) | 65 (0) | 63 | 197 | 77 | 96 |
| 3 | 35 (0) | 45 (0) | 75 (0) | 50 (-a) | 32 | 168 | 46 | 72 |
| 4 | 26 (-1) | 35 (-1) | 100 (1) | 56 (-1) | 47 | 159 | 37 | 66 |
| 5 | 35 (0) | 45 (0) | 75 (0) | 65 (0) | 62 | 193 | 76 | 97 |
| 6 | 35 (0) | 45 (0) | 75 (0) | 65 (0) | 62 | 192 | 76 | 99 |
| 7 | 50 (+a) | 45 (0) | 75 (0) | 65 (0) | 58 | 156 | 35 | 67 |
| 8 | 26 (-1) | 54 (1) | 45 (-1) | 75 (1) | 64 | 143 | 29 | 58 |
| 9 | 35 (0) | 45 (0) | 30 (-a) | 65 (0) | 61 | 178 | 57 | 89 |
| 10 | 35 (0) | 45 (0) | 120 (+a) | 65 (0) | 49 | 185 | 64 | 88 |
| 11 | 35 (0) | 60 (+a) | 75 (0) | 65 (0) | 56 | 154 | 34 | 63 |
| 12 | 26 (-1) | 35 (-1) | 45 (-1) | 56 (-1) | 37 | 139 | 19 | 52 |
| 13 | 44 (1) | 35 (-1) | 45 (0) | 75 (1) | 48 | 183 | 46 | 85 |
| 14 | 35 (0) | 45 (0) | 75 (0) | 65 (0) | 61 | 194 | 76 | 96 |
| 15 | 35 (0) | 45 (0) | 75 (0) | 80 (+a) | 40 | 177 | 55 | 86 |
| 16 | 44 (1) | 35 (-1) | 100 | 75 (1) | 46 | 174 | 43 | 82 |
| 17 | 35 (0) | 45 (0) | 75 (0) | 65 (0) | 62 | 195 | 72 | 93 |
| 18 | 44 (1) | 54 (1) | 45 (-1) | 56 (-1) | 51 | 150 | 34 | 62 |
| 19 | 35 (0) | 30 (-a) | 75 (0) | 65 (0) | 50 | 148 | 30 | 61 |
| 20 | 26 (-1) | 54 (1) | 100 (1) | 75 (1) | 33 | 160 | 36 | 73 |
| 21 | 20 (-a) | 45 (0) | 75 (0) | 65 (0) | 44 | 171 | 50 | 69 |
| 22 | Conventional solvent extraction | |||||||
Table 1. Enzyme pre-treatment conditions (actual and coded) investigated and corresponding responses observed; Coded values “0”,“+1and -1” and “+a and –a” indicated center, axial and factorial points respectively K, L, M and N are responses measured in g/100 g, mgGAE/g, µmol TE/g and µg/mL (IC50).
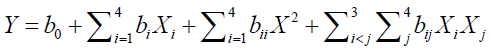
Where terms Y and responses to be optimized are, intercept (b), linear effect variables (bX), quadratic effect (bX2) and interactions (XiJi)
Extraction procedure
For enzyme assisted solvent extraction (EASE), 5 g of pomegranate peel was mixed with 10 mL of buffer of required pH and blended with enzyme under the conditions as mentioned in Table 1. The resultant mixture was heated at 90ºC to deactivate the enzyme cocktail. For comparison, a same amount of powdered pomegranate peel was diluted with 10 mL phosphate buffer of pH 7, incubated at 37°C for 120 min. The liberated polyphenols were collected by shaking with 80% aqueous ethanol in an orbital shaker (Gallenkamp, UK) for 1 hour. All the extracts obtained were concentrated using a Rotary Evaporator (EYELA, N-N series, Tokyo, Japan) under reduced pressure. The dried extracts were weighed to calculate percent yield and stored at -4°C until used for analysis.
Total phenols concentration (TPC)
TPC was assessed using Folin-Ciocalteu reagent [19] with modifications. Briefly, 5 mg extract and various concentrations of gallic acid (10–200 ppm) were separately mixed with 1.5 mL deionized water and treated with 50 μL of 20% Folin-Ciocalteu reagent. The aliquots were mixed with 300 μL of 20% sodium carbonate (w/v), incubated at 40ºC for 20 min, chilled in an ice bath and a portion (200 μL) was transferred to 96 well plate. The absorbance was measured at 755 nm using 96 well plate readers (Biotek-MQX-200, Biotek Ind., Highland park, USA). Absorbance of various gallic acid concentrations were plotted to get calibration curve (R2=0.9986) and phenolic contents were calculated as milligram gallic acid equivalents (mg GAE)/g of pomegranate peel extract.
HPLC analysis of phenolic compounds
Extracts of pomegranate peel were analyzed by RP-HPLC as described previously with modification [20] using a diode array detector (RP-HPLC-DAD). Briefly, 50 mg of crude extract was dissolved in 5 mL of acidified methanol (1% v/v HCl), and refluxed at 95ºC for 90 min. BHT was added (0.5 mg/mL) as preservative antioxidant. The resultant solution was centrifuged at 5000 rpm for 10 min; the upper layer was sonicated for 5 min to remove air bubbles. Extracts were passed through a 0.45 μm (Millipore) filter and subjected to
using a Shimdadzu HPLC system LC-10A, fitted with a DAD (G1315B DAD) detector and Shim-Pack CLC-ODS C-18 column (250 × 4.6 mm; 5 μ particle size; Merck Darmstadt, Germany). Two solvents (A & B) were used for gradient elution. Solvent A (94:6 v/s water-acetic acid mixture) and solvent B (acetonitrile, 100%). The solvent elution profile involved a gradient 0-15 min 15% B, 15-30 min 45% B and 30-45 min 100% B. Peak elution was monitored at 280 nm and quantified using CSW32 (dataapex) Chromatography Station/ data handling software.
DPPH• scavenging assay
Previously reported radical scavenging assay [21] was modified in order to make it applicable at micro level. Briefly, 110 μL of 1000, 100, 10 and 1 ppm extract was mixed with 100 μL of freshly prepared DPPH solution (1000 ppm) in 96 well plates. The plates were incubated at 35°C for 15 min and then absorbance (A) was measured at 517 nm using 96 well plate reader (Biotek- MQX-200, Biotek Ind., Highland park, USA).
The half-maximal inhibitory dose of extracts (IC50) was obtained from a plot of percentage inhibition verses various concentrations of pomegranate peel extracts.
Trolox equivalent antioxidant capacity assay (TEAC)
The antioxidant potential of extracts was assessed in terms of TEAC following previously described method [16]. Trolox was used as positive controls and antioxidant potential was expressed as μmol of trolox/g of pomegranate peel extract.
Statistical analysis
Analysis of the experimental design for each response was carried out using Design Expert (version 10, Stat-Ease, Inc., Minneapolis, USA) to investigate the effect of Enzyme concentration, temperature, incubation time and pH and interactions among them. Analysis of variance (ANOVA) was applied to screen out significant (p<0.05) and non-significant (p>0.05) terms. After carrying out ANOVA, the non-significant terms were deleted from the second-order polynomial equation for better accuracy. From these refined equations, three dimensional (3-D) response surfaces were plotted to illustrate and discuss the interactions between two any factors. Similarly, certain other tests (lack of fit, variation co-efficient and co-efficient of determination) were also observed to evaluate Model Adequacy. Finally, validation experiments were conducted to verify the validity of the statistical experimental design.
Model adequacy check
Preliminary investigation indicated that treatment with a cocktail enzyme mixture comprising cellulases, pectinase and protease (50:25:25) improved weight of extractable material from peel (extract yield) as compared to other enzyme formulation. Further investigations were extended to factorial (coded values -1 and +1) and axial points (coded values –α and +α) in a rotatable central composite design to get insight into interactions and quadratic effects of pre-treatment parameters (Table 1). One of the reliable ways to check the fitness of applied model is analysis of variance (ANOVA). ANOVA compares variation due to changes in treatment conditions with the variation contributed by indeterminate errors. A probability (p) value <0.05 indicate that applied design fits well.
Another way to check the model fitness is using a “lack of fit” test. A non-significant (p>0.05) lack fit probability (Table 2) is an indication that selected model fitted well to the given experimental conditions. The low coefficients of variation (CVs) values of 0.97- 3.35% verified that results are reliable and reproducible [22]. Therefore, all the responses including extract yield, TPC, TEAC and RSC, were generated following multiple regression model. The parameters (EC, T, pH and t) or their interactions imparting non-significant (p<0.05) effects were deleted from the finally modified equations (1-4). The terms having positive signs significantly (p<0.05) increased the responses (extract yield, TPC, TEAC and RSC) while terms followed by minus sign caused decrease in particular response; in the relations expressed below A = enzyme concentration, B= temperature, C=reaction time , D=reaction pH.
| Yield (g/100g of dry weight) | TPC (mg GAE/g of extract) | |||||
|---|---|---|---|---|---|---|
| Source | MS | F-ratio | P-Value* | MS | F-ratio | P-Value* |
| Model | 16365 | 6081 | 000 | 53035 | 19512 | 000 |
| Linear | ||||||
| A-EC | 9800 | 3642 | 000 | 11250 | 4139 | 000 |
| B-T | 1800 | 669 | 003 | 1800 | 662 | 005 |
| C-t | 25646 | 9530 | 004 | 7864 | 2893 | 000 |
| D-pH | 3200 | 1189 | 001 | 4050 | 1490 | 001 |
| Interaction | ||||||
| AB | 020 | 007 | 079 | 11729 | 4315 | 000 |
| AC | 113 | 042 | 054 | 35113 | 12918 | 000 |
| AD | 445 | 165 | 025 | 27797 | 10227 | 000 |
| BC | 37813 | 14051 | 000 | 013 | 005 | 084 |
| BD | 6091 | 2263 | 000 | 37124 | 13659 | 000 |
| CD | 9113 | 3386 | 000 | 313 | 115 | 032 |
| Quadratic | ||||||
| A2 | 18007 | 6691 | 000 | 172693 | 63536 | 000 |
| B2 | 11418 | 4243 | 000 | 343873 | 126516 | 000 |
| C2 | 6323 | 2350 | 000 | 28746 | 10576 | 000 |
| D2 | 115058 | 42756 | 000 | 85589 | 31489 | 000 |
| Lack of fit | 707 | 1414 | 054 | 075 | 020 | 082 |
| R2 | 09930 | 09978 | ||||
| Adj R2 | 09766 | 09927 | ||||
| CV (%) | 325 | 097 | ||||
Table 2. Analysis of Variance (ANOVA) for yield and total phenols concentration for pomegranate peel *The probability (p) value <005 indicated statistically significant terms, TPC=total phenols concentration.
A large coefficient of determination (R2), observed for extract yield (0.9766), TPC (0.9978), TEAC (0.9976) and DPPH (0.9939) indicates that chosen model fits the responses under given experimental conditions. Similarly, adjusted R2 larger than 95% (0.95) confirms good agreement between predicted and observed values of these responses. The multiple regression equations (1-4) give interesting detail on the effect of independent variables as can be seen from the magnitude and sign (+ve or –ve) for the coefficients. For instance, interactions terms BC (temperature x time), CD (time x pH) had predicted negative impact on extraction yield and Table 2 shows such interactions would be significant (P<0.05). Notice that other possible interactions terms (AB, AC, & AD) are predicted to have insignificant effects on extraction yield (Table 2) and therefore do not appear in Equation (1).
Effect of enzyme concentration (EC; A)
From the assembled results for ANOVA (Tables 2 and 3) and multiple regression equations (1-4) it was evident that EC (term “A” in equation 1) had a significant (p<0.05) positive effect towards extract yield whilst decreasing the antioxidant quality of extracts (TPC, TEAC and DPPH); similar interaction have been previously [23]. The interaction between EC and temperature, pH and incubation time was expressed using three-dimensional plot (Figure 1). Figure 1a is plot of extract yield (g/100 g) with variation in EC and temperature while keeping other two parameters (incubation time and pH) at most feasible level. It is clear from Figures 1a and 1b that EC up to 3.5%, temperature equal to 45ÃÂC and incubation time of 65 minutes sharply increased extract yield while further increase in these parameters did not cause parallel effect.
| TEAC(µmol TE/g of extract) | DPPHRSC (IC50µg/mL) | |||||
|---|---|---|---|---|---|---|
| Source | MS | F-ratio | P-Value | MS | F-ratio | P-Value |
| Model | 47881 | 17663 | 000 | 36454 | 6933 | 000 |
| Linear | ||||||
| A-EC | 11250 | 4150 | 000 | 155 | 029 | 061 |
| B-T | 800 | 295 | 014 | 155 | 029 | 061 |
| C-t | 6785 | 2503 | 000 | 671 | 128 | 030 |
| D-pH | 4050 | 1494 | 001 | 10139 | 1928 | 000 |
| Interactions | ||||||
| AB | 902 | 333 | 012 | 7280 | 1385 | 001 |
| AC | 13289 | 4902 | 000 | 28306 | 5384 | 000 |
| AD | 3532 | 1303 | 001 | 12165 | 2314 | 000 |
| BC | 1986 | 733 | 004 | 1707 | 325 | 012 |
| BD | 23292 | 8592 | 000 | 4810 | 915 | 002 |
| CD | 1103 | 407 | 009 | 2193 | 417 | 009 |
| Quadratic | ||||||
| A2 | 197861 | 72991 | 000 | 142731 | 27147 | 000 |
| B2 | 346126 | 127686 | 000 | 215840 | 41052 | 000 |
| C2 | 39522 | 14580 | 000 | 9502 | 1807 | 001 |
| D2 | 112544 | 41517 | 000 | 53176 | 10114 | 000 |
| Lack of fit | 013 | 003 | 097 | 637 | 136 | 036 |
| R2 | 09976 | 09939 | ||||
| Adj R2 | 09920 | 09795 | ||||
| CV (%) | 335 | 300 | ||||
*The probability (p) value <005 indicated statistically significant terms
Table 3. Analysis of Variance (ANOVA) for TEAC values for pomegranate peel extracts.
Effect of temperature (B)
The probability columns (Tables 2 and 3) indicate that linear effect of temperature (B) was significant (p<0.05) towards extract yield and TPC but not towards TEAC and DPPH. This trend indicates that the structure and activity of enzymes is sensitive towards temperature. However, the antioxidant capacity of liberated polyphenols was not affected by temperature i.e. 45-70°C [24]. Interestingly, temperature interactions with other parameters (cf. BC and BD, BA and BD, BC and BD, and BA and BD in Tables 2 and 3) were predicted to (p<0.05) affect extract yield, TPC, TEAC and DPPH. Synergisms are evidenced by a sharp slope in the 3-dimensional plot of extract yield (g/100 g), EC and temperatures up to 50ºC (Figure 1a). Interestingly, the plateau in Figures 1d and 1e at 50ºC indicated that rising temperature would decrease extract yield. Similar kinds of behaviour have been predicted for for other TPC, TEAC and DPPH (equations 3 and 4).
Effect of pH and incubation time
The effect of pH (D) and incubation time (C) towards the liberation of polyphenols and their antioxidant characteristics can be easily estimated from analysis of variance data (Tables 2 and 3) and subsequent multiple regression equations (1-4). A significant effect of pH and incubation time was observed (p<0.05) for all the responses investigated except DPPH radical scavenging capacity. The sharp curvature in Figures 1e and 1f disclosed that pH majorly controlled the liberation of polyphenols. Slight shift in pH below or above 6.5 had produced a marked decrease in extract yield possibly because enzymes are highly sensitive towards pH change [25]. In Figures 1d and 1f, further extending the incubation time beyond 58 min did not improve extract yield. Multiple regression equations (1-4) indicate incubation time (C) affected all responses positively but the effect of pH could be positive or negative. Similarly, quadratic effect of these parameters caused a decrease in observed responses.
Optimization of conditions and their validation
The observed results showed differences in all the responses for any predicted set conditions. For example, increases in enzyme concentration (EC) improved extract yield but inversely affected antioxidant behavior. Mild acidic conditions retained antioxidant characteristic (Eqs. 1-4) but decreased extract yield. These evidences demand an alternative optimization approach producing optimal extract yield with substantial amounts of total phenolic content and ample level of in vitro antioxidant capacities.
In the present case, all the responses were set to maximize but the value for DPPH inhibition was set to be minimized (IC50). The resultant solution of high desirability (0.9) has been plotted in Figures 2 and 2b. The central contour (desirability>0.9) indicate the most suitable experimental conditions which can produce good quantity of pomegranate peel extracts without compromising the levels of phenolics and antioxidant character (Figure 3). Overall, the solution with maximum desirability (0.9) forecasted that pretreatment of pomegranate peel with 3.8% of enzyme cocktail at 6.7 pH and 49ºC for 85 minutes would produce 67.44 g/100 g of pomegranate peel extracts containing 297 mg GAE/g (~29% w/w) total polyphenols with 394 μmol TE/g and 7365 μg/mL RSC (IC50).
A new set of experiments were conducted to verify the optimum conditions and observed results (Table 4) in this context, authenticated that the optimum conditions predicted by the model are inline agreed with actual results observed. When compared with conventional solvent extraction EASE improved extract yield by three and half folds (Table 4) as well as total phenols content and ABTS radical quenching value. However, the radical quenching activity for EASE samples were decreased as measured by IC50 (concentration that inhibits 50% of DPPH).
| Sr No | Variables and their optimum level | Responses observed | ||||||
|---|---|---|---|---|---|---|---|---|
| EC | pH | T (ºc) | t (Min) | YieldK | TPCL | TEACM | DPPHN | |
| (A) | (C) | (D) | (E) | |||||
| 1 | 38 | 67 | 41 | 85 | 6644 | 27774 | 39974 | 7365 |
| 2 | 38 | 67 | 41 | 85 | 6859 | 28420 | 40112 | 7236 |
| 3 | 38 | 67 | 41 | 85 | 6401 | 27185 | 39526 | 7344 |
| Expt average | 6589 ± 264 | 27793 ± 617 | 39870 ± 306 | 7315 ± 069 | ||||
| Results predicted with desirability (0957) | 6744 | 297 | 394 | 7365 | ||||
| Control (no enzyme pre-treatment) | 1891 ± 064 | 15301 ± 186 | 11825 ± 414 | 2677 ± 937 | ||||
Table 4. The detail of validation experiments conducted against most desirable EASE experimental conditions for pomegranate peel polyphenol extraction; Responses observed - upper case letters express (K) g/100 g, (L) mgGAE/g, (M) μmol TE/g, (N) μg/mL (IC50).
Composition of pomegranate extract
Analysis by RP-HPLC-DAD data revealed (Table 5) the presence of a range of phenolic constituents similarly to those described elsewhere [18] notably, vanillic acid, ferulic acid, chlorogenic acid and Syringic acid. Chlorogenic acid (a potent antidiabetic), ferulic and vanillic acid (anti-cancers and chemo preventives) established that pomegranate peel extracts might be a source of phenolic acids for value addition in food and pharmaceutical industries [2-4].
| Sr No | Retention Time | Phenolics | Max Conc |
|---|---|---|---|
| (min) | (µg/g of extract) | ||
| 6 | 1107 | Vanillic Acid | 34101 |
| 8 | 1802 | Ferulic Acid | 18183 |
| 5 | 1022 | Chlorogenic Acid | 12704 |
| 3 | 794 | Caffeic Acid | 7519 |
| 7 | 1605 | Syringic Acid | 5103 |
| 2 | 409 | Quercitin | 4047 |
| 4 | 895 | Myricetine | 3515 |
| 10 | 2201 | M-Coumeric Acid | 1496 |
| 1 | 348 | Gallic Acid | 1415 |
| 9 | 2051 | P-Coumeric Acid | 713 |
| 1922 | Sinapic Acid | ND |
Table 5. Phenolics compounds authenticated in pomegranate peel extracts obtained by EASE; ND: not detected
This paper provides a comprehensive insight into multi-response optimization of enzyme assisted solvent extraction (EASE) of antioxidant phenolics from pomegranate peel (pomegranate peel). It was observed that increases in enzyme concentration may enhance extract yield, total phenols content of extracts, and free radical quenching characteristics expressed with regards to ABTS. HPLC-DAD analysis revealed the presence of phenolic acids as major components and authenticated that optimized enzyme pre-treatment does not deteriorate or degrade constitutional phenolics.
The present work was partially funded by Higher Education Commission of Pakistan under Ph.D. Indigenous Fellowship Program. A UC Davis fellowship to SB is acknowledged.