ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Ningthoujam Manikar1, Satyendra Kumar1, Khalid Habib1 & Tasneem Fatma2
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Cyanobacteria contribute significantly to the maintenance of flooded rice field fertility. In this investigation, the protective effect of osmolytes and antioxidative enzymes was studied on the paddy field cyanobacterial biofertilizer; Anabaena variabilis exposed to Malathion pesticide. With increasing concentration of malathion the test organism showed increased free radical mediated toxicity. The activity of antioxidative enzymes superoxide dismutase (SOD), catalase (CAT), and ascorbate peroxidase (APX) enhanced considerably. Interestingly, osmolytes â proline, sucrose, mannitol, trehalose and glycogen levels also increased under the malathion treatment indicating their involvement of this osmoprotectants in a free radical scavenging mechanism. Taken together, our results indicate a probable role of osmolytes in co-operation with antioxidative enzymes in lowering pesticide induced oxidative stress.
Keywords |
| Malathion, Anabaena variabilis, Antioxidative enzymes, Osmolytes |
INTRODUCTION |
| An indispensable requirement of sustainable agriculture is the continuous renewal of soil structure and fertility with renewable resources, lessening the need for chemical fertilizers, thus lessening their environmental burden. The most promising ones are the wild type and transgenic N2–fixing cyanobacteria and bacteria, which have the great potential as a source of biofertilizer. Cyanobacteria, the oxygen producing photosynthetic prokaryotes grow profusely in rice fields which provide a suitable environment for the luxuriant growth of these organisms. Application of diazotrophic cyanobacteria, Anabaena variabilis, as biofertilizer for rice cultivation has a beneficial effect on crop productivity and maintenance of soil fertility [58]. Modern intensively managed agricultural system has an intrinsic reliance on the use of chemical pesticides. Pesticides adversely affect non target organisms and are widely used in agricultural ecosystems, in which cyanobacteria, the non target species, are major components of the wetland biota. Thus, cyanobacteria living in agricultural ecosystems and wetlands are exposed to these pesticides. Although pesticides are indispensable to modern agricultural practice, use of these pesticides over the years has resulted in problems caused by their interactions with biological systems in the environment and had deleterious effects on algae by influencing soil algal growth, photosynthesis, nitrogen fixation, biochemical composition and metabolic activities [48]. |
| Malathion is one such non-systemic, wide spectrum organophosphate insecticide used in agricultural as well as nonagricultural purposes [50]. It is one of the five most commonly used pesticides in India, accounting for 65% of all organophosphate pesticides applied in the field [32]. There are reports that malathion adversely affects various processes in different groups of algae [5, 20, 62, 63]. Malathion shares a common mechanism of toxicity by affecting the nervous system via cholinesterase inhibition. The primary site of action of the organophosphorus insecticides is the enzyme acetylcholinerase (AChE) in insects and other living organisms[ 9]. |
| Pesticide induced oxidative stress has been a focus of toxicological research for the last decade as a possible mechanism of toxicity. Pesticide induced oxidative stress accelerates lipid peroxidation, thereby affecting structural integrity and permeability of cellular membranes [30]. Oxidative damage in living organisms, including plants and cyanobacteria, is characterized by the enhanced production of reactive oxygen species (ROS). ROS can damage all cellular macromolecules and can interact with proteins, pigments, carbohydrates, lipids, and nucleic acids, resulting in dramatically reduced growth and productivity, abnormal development and eventually death and molecular damage in plants [53]. Malondialdehyde is a secondary end product of polyunsaturated fatty acid oxidation, and is widely used as an indicator of oxidative stress [38]. Therefore, we hypothesizes that malathion like most environmental stresses, induces ROS production in A. variabilis causing oxidative damage. To be able to endure oxidative damage under conditions which favor increased oxidative stress, every cyanobacterial cell possesses a complex array of enzymatic antioxidant defense systems comprising mainly the enzymes SOD, CAT, and APX [6]. SOD is the first enzyme of the enzymatic antioxidative pathway that catalyses the dismutation of O2ÃÂ to H2O2 and O2 [35]. The enzymes CAT and APX subsequently detoxify H2O2. To test this hypothesis, an attempt has been made to study the effects of malathion causing cellular damage in A. variabilis by measuring enzymatic antioxidants (SOD, CAT, and APX). |
| Many organisms cope with osmotic stress by synthesizing and accumulating some compatible solutes, which are termed osmoprotectants or osmolytes. These compounds are small, electrically neutral molecules, which are nontoxic even at high molar concentrations. These include amino acids such as proline; quaternary ammonium compounds such as glycinebetaine; diverse sugar alcohols, and sugars (e.g., sorbitol, sucrose, and trehalose) etc. [59]. Generally they protect the plants from stress through different courses such as cellular osmotic adjustment, detoxification of reactive oxygen species, protection of membrane integrity, and stabilization of enzymes and proteins [13]. Cyanobacteria switch the production of one organic compound to another with changing environmental conditions [66] the end products may be osmolytes. The aim of the present is to explore the cyanobacterial strain, Anabaena variabilis commonly used as biofertilizer in paddy fields in terms of its pesticide tolerance with special reference to oxidative stress and osmolyte accumulation. |
MATERIALS AND METHODS |
A. Organism and culture conditions |
| The diazotrophic axenic culture of A. variabilis was procured from the National Center For Conservation And Utilization of Blue-Green Algae, Indian Agricultural Research Institute, New Delhi. The culture was grown in BG- 11 medium at pH 7.3, devoid of nitrogen source[ 61]. Flasks and media were sterilized in an autoclave (Yorco, India) maintaining 1 Kg cm-2 pressure for 15 min. The cultures were grown in 500 mL flasks containing 200 mL of medium; maintained in an air-conditioned culture room at 30±1ï°C. The room was maintained under aseptic conditions and was illuminated with fluorescent tubes providing light intensity of 25μ mol m-2 s-1 around the culture vessels, in alternating 12 h light and 12 h dark cycles under aseptic conditions. The cultures were regularly hand shaken to maintain homogeneity. Stock solution of the pesticide was prepared in sterilized double-distilled water. For evaluating pesticide toxicity, malathion (50% E.C.) was separately added aseptically to the fresh medium in calculated amounts to obtain a final concentration of 25, 50, 75 and 100 μg mL-1. For ‘control’ sets test microorganisms were grown without adding the pesticide. Preliminary experiments were carried out to determine the suitable range of pesticide concentrations. The biomass was harvested by filtration through fine nylon cloth and washed twice with distilled water to remove the remaining pesticide. Biochemical analysis of 20 day old biomass of test organisms grown under stress and control conditions was carried out in triplicate for estimation of carbohydrate, MDA, H2O2, SOD, CAT, APX, proline, mannitol, trehalose, glycogen and sucrose. |
| B. Chemicals |
| Commercial grade Malathion (50% E.C.) was obtained from Bharat Rasayan Limited, New-Delhi (India). The molecular formula and chemical name for malathion is C10H19O6PS2 and O, O-dimethyl phosphorodithioate of diethyl mercaptosuccinate. All other chemicals used were of analytical grade purchased from Merck India Pvt. Ltd., and HiMedia Laboratories Pvt. Limited, Mumbai (India). |
| C. Estimation of growth measurement |
| The growth of the malathion treated A. variabilis was monitored as dried biomass estimation at 4-day intervals up to 24 days. Stress was applied as 0, 25, 50, 75 and 100 μg mL-1 of malathion. The overnight oven dried (60°) biomass was expressed as mg mL-1. Specific growth rate was estimated as biomass yield as described by[ 68]. Specific growth rate (μ) is defined as the derivative of population with respect to time, over population |
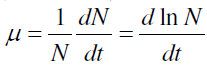 |
| where N and t denote population and time, respectively. |
| D. Estimation of protein and carbohydrate |
| Protein was determined with Folin phenol reagent [39]. 1 mg of algal biomass was taken in a test tube and 1 mL 1N NaOH was added. The test tube was placed in a boiling water bath for 10 min. 5 mL of reagent A (prepared by adding 1 mL freshly prepared 1% Na-K tartarate solution containing 0.5% CuSO4 into 50 mL 2% Na2CO3 solution) and incubated at room temperature for 10 min. 0.5 mL reagent B (Folin reagent) was added, mixed properly at the shaker and again incubated at room temperature for 30 min. The absorbance of the supernatant was read at 650 nm against Folin reagent as blank. The protein content was expressed as mg g-1 of dry weight of the cultures. |
| Carbohydrate production was estimated using anthrone reagent method[ 60]. 1 mg of dry algal biomass was taken in a test tube and 1.25 mL of double distilled water was added. 4 mL of anthrone reagent were added to 1.25 mL each of blank, standard and sample solutions, incubated in boiling water for 8 min and then cooled for 10 min at room temperature. Absorbance of standard and sample solutions was noted at 620 nm against blank. Carbohydrate was expressed as mg g-1 of dry weight of the culture. |
| E. Estimation of Lipid Peroxides |
| Lipid peroxidation was determined by measuring MDA using the thiobarbituric acid (TBA) method [31]. 25 mg algal biomass was homogenized in 1.5 mL 0f 1% trichloroacetic acid (TCA) and centrifuged at 10,174 g for] 5 min at 4ï°C. To 1 mL of the supernatant, 4 mL of 0.5% TBA was added. The mixture was heated at 95ºC for 30 min and then quickly cooled in an ice bath. After centrifugation at 2543 g for 10 min at 4ï°C the absorbance was read at 532 nm and correction for specific turbidity was done by subtracting the absorbance at 600 nm. A total of 0.5% TBA in 20% TCA served as blank. The MDA content was calculated according to its extinction coefficient of 155 mM-1 cm- 1. |
| F. Estimation of Hydrogen Peroxide |
| H2O2 levels were determined according to the method of Jana and Choudhuri [33]. 50 mg fresh biomass was homogenized in 1.5 mL of sodium phosphate buffer (50 mM, pH 6.5). The homogenate was centrifuged at 10,174 g for 15 min. H2O2 was determined by mixing 250 μL of the supernatant with 750 μL of 0.1% titanium chloride in 20% (v/v) H2SO4. After 1 min, the intensity of the developed yellow color was measured at 410 nm. Levels of H2O2 were calculated using the extinction coefficient of 0.28 μmol cm-1. |
| G. Estimation of Antioxidative Enzyme Activity |
| SOD activity was assayed spectrophotometrically [21]. 50 mg fresh biomass was homogenized in 2 mL 0.5 M phosphate buffer (pH 7.5), 3 mM EDTA, 1% PVP and 1% Triton X 100 under cold condition. The homogenate was centrifuged at 22, 891 g at 4ºC. The supernatant was used for the enzyme assay. SOD activity was assayed by monitoring the inhibition of photochemical reduction of nitroblue tetrazolium chloride (NBT), using a reaction mixture consisting of 1 M Na2CO3, 200 mM methionine, 2.25 mM NBT, 3 mM EDTA, 60 μM riboflavin and 0.1 M phosphate buffer (pH 7.8). One unit of SOD was defined as the amount of enzyme that inhibits 50% NBT photoreduction. SOD was expressed as unit of enzyme mg-1 protein h-1. |
| CAT activity was assayed by measuring the initial rate of disappearance of H2O2 (Aebi 1984). 50 mg of fresh biomass was taken and homogenized with 2 mL of extraction buffer (0.5 M phosphate buffer, pH 7.5, 3 mM EDTA, 1% PVP and 1% Triton X 100). The homogenate was centrifuged (at 10 174 g for 20 min) and the supernatant was separated for enzyme assay. To 100 μL of enzyme extract, 1.6 mL phosphate buffer, 0.2 mL 0.3% H2O2 and 3 mM EDTA was added in a test tube. The reaction was allowed to run for 3 min. The absorbance of the supernatant was observed at 240 nm against blank. Enzyme activity was calculated by using extinction coefficient 0.036 mM-1 cm-1 and was expressed in nkat mg protein-1 (katal = conversion rate of one mol substrate per second). |
| APX activity was determined according to Nakano and Asada[ 43]. 50 mg of fresh biomass was homogenized with 2 mL of extraction buffer (0.5 M phosphate buffer, pH 7.5, 3 mM EDTA, 1% PVP and 1% Triton X 100). The homogenate was centrifuged (at 10,174 g for 20 min) and the supernatant was separated for enzyme assay. The reaction mixture contained 0.5 mM Na-phosphate buffer (pH 7.5), 0.5 mM ascorbate, 3.0 mM EDTA, 1.2 mM H2O2, and 0.1 mL enzyme extract in a final assay volume of 1mL. Ascorbate oxidation was read at 290 nm. The concentration of oxidized ascorbate was calculated using extinction coefficient (ε = 2.8mM cm-1) and was expressed in nkat mg protein-1. |
| H. Estimation of Osmolytes |
| Free proline content in the algal biomass was determined following the method of Bates et al. [10]. Wet algal biomass (50 mg) was homogenized in 10 mL of sulphosalicylic acid (3%). About 2 mL of extract was taken in test tube and to it 2 mL of glacial acetic acid and 2 mL of ninhydrin reagents were added. The reaction mixture was boiled in water bath at 100ï°C for 30 min. After cooling the reaction mixture, 4 mL of toluene was added. After thorough mixing, the upper organic toluene layer (containing the chromophore) was aspirated from the aqueous phase and absorbance read at 520 nm using toluene as blank. Concentration of proline was estimated by referring to a standard curve of proline. The amount of proline in the sample was calculated in μg (proline) g-1 dry weight of samples. |
| Mannitol was determined by the Mannitol dehydrogenase (MDH) activity [27]. One unit of MDH activity was defined as the amount of enzyme which catalyzes the reduction of 1μmole of NAD+ per min at 23°C and pH 10.5. To two test tubes 1.4mL glycine buffer (100mM; pH10.5), mannitol (500mM, 0.2mL) and NAD (0.12g dissolved in 5mL of de-ionized water; 0.2mL) were added. For the blank, 0.2mL water added and the mixture immediately stirred on a vortex mixer, added to a 1cm cuvette and placed in a spectrophotometer. For the test algal sample, 0.2mL of 10,000X diluted MDH was added, vortex stirred then immediately added to a cuvette and absorbance was measured at 340nm after 0, 1,2,3,4, and 5 min. Increase in absorbance per min between 2 and 3 min was used for the activity calculation based on Beer’s Law: |
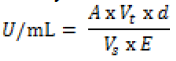 |
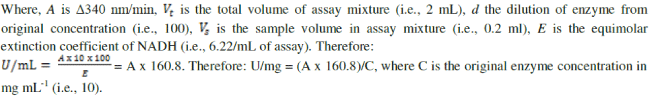 |
| Sucrose was determined in algal biomass using anthrone reagent [64]. 10 mg dried sample was homogenized in 10 mL 80% ethanol. Hexoses were destroyed by placing the reaction tubes in boiling water for 10 min. After cooling, 0.1 mL 30% aqueous KOH was added and kept at 100ºC for 10 min. When the tubes were cooled to room temperature, 3 mL anthrone reagent was added and incubated at 38ºC for 20 min and absorbance was recorded at 620 nm spectrophotometrically taking sucrose as standard. Amount of sucrose was expressed as μg g-1 dry weight of samples. |
| Trehalose and glycogen were estimated following the method of Parrou and François [49]. Fresh biomass (500 mg) was collected by centrifugation (3 min at 5000 g , 0-4°C), resuspended in 0.25 mL of 0.25 M Na2CO3 using screw Eppendorf tubes, and incubated at 95°C for 4 h. The mixture is brought to pH 5.2 by addition of 0.15 mL of 1 M acetic acid and 0.6 mL of 0.2 M Na-acetate, pH 5.2. One-half of the suspension is incubated overnight with trehalase (0.05 U/mL) at 37°C, and the second half with A. niger amyloglucosidase preparation (1.2 U/mL) at 57°C, under constant agitation (for instance in a hybridization chamber). The suspensions are centrifuged for 3 min at 5000: glucose is determined on 20 μL (adequately diluted in water) of supernatant by addition of 200 μL of glucose oxidase mixture and read at 420 nm in an ELISA reader apparatus. |
| I. Statistical analysis |
| The experimental data values were tabulated as the mean ±standard error (SE) of three replicates. Tests of significance were performed by one-way analysis of variance (ANOVA) followed by Dunnett’s test with control (P*<0.01, P**<0.001 and P***<0.0001 and ns for not significant respectively) by GraphPad Prism Version 5.00 for Windows (GraphPad Software, San Diego, CA, USA). |
RESULTS AND DISCUSSIONS |
| A. Effect of malathion on growth |
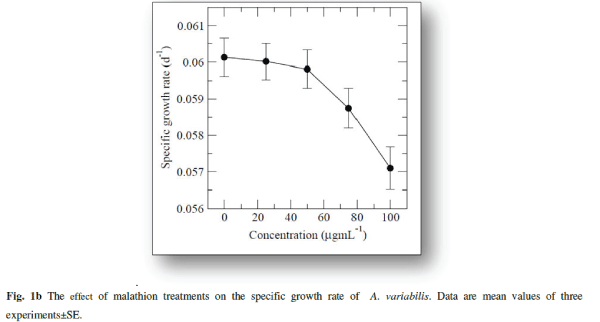
| The cyanobacterium A.variabilis showed inhibitory growth response against the insecticide malathion being 28.82% (p<0.001), 48.08% (p<0.0001), 57.5% (p<0.0001) and 63.97% (p<0.0001) at 25, 50, 75 and 100 μg mL-1 as compared to control after 20 days of treatment (Fig. 1a). A steady-state growth pattern was observed in the control sample up to a period of 20 days (Fig.1a). |
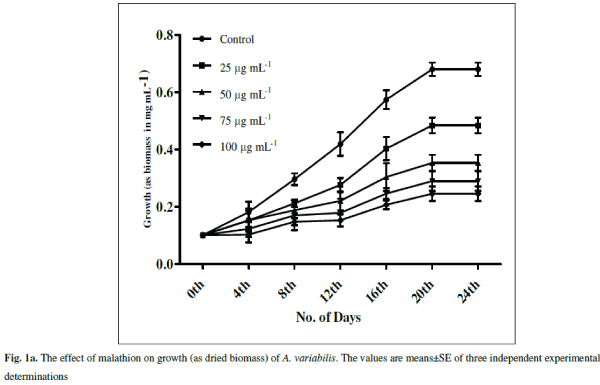 |
| With increasing concentrations inhibitory effects were more pronounced with decreasing specific growth as shown in Fig. 1b. Reduction in the growth rate of cyanobacteria in presence of pesticide may be due to a decrease in photosynthesis especially in chlorophyll-a 1 which subsequently leads to several secondary effects. Similar inhibitory effect on growth of cyanobacteria with other insecticides, endosulfan, was correlated with the inhibition in chlorophyll synthesis and photosynthetic activity in Plectonema boryanum, Aulosira fertilissima, Anabaena variabilis and Nostoc muscorum [51, 36]. Higher toxic effects at higher malathion doses may be attributed to the permeability of the pesticide across the cell membrane of A. variabilis due to the presence of thin and diffluent mucilage in A. variabilis [2]. The effect of malathion on cyanobacterial and algal populations has been reported to be low at low concentrations whereas inhibitory at high doses [ 62, 67] |
 |
| B. Effect of malathion on protein and carbohydrate contents |
| Malathion had a significant effect on protein content which increased till 50 μg mL-1 malathion compared to control being 31% (P<0.0001) and 48% (P<0.0001) respectively (Fig. 2a). At low concentrations of pesticide, increase in protein content suggests that the pesticide stimulates synthesis of stress retarding proteins [36]. Almost all kinds of stress induce gene expression and synthesis of ‘‘heat-shock proteins’’ (Hsps), ‘‘stress-induced proteins’’ or ‘‘stress proteins’’ in cells subjected to stress [19, 25]. Methomyl (a carbamate insecticide) is reported to induce synthesis of Hsps in Zea mays L. [16]. Stress alleviation in cyanobacteria has been known to be achieved through the production of stress proteins. They are known to synthesize a variety of proteins in response to various stresses [34]. In stressed cyanobacterial cells, protein synthesis patterns were usually modified leading to reduced synthesis of most normal cell proteins and to an induced or enhanced synthesis of a set of special stress proteins. Such stress proteins were detected in protein labeling experiments after heat shock [12, 14], dessication [26, 56] nitrogen deficiency [22], impairment of DNA-synthesis [ 44 ] and salt stress [12, 28] . Further increase in malathion resulted in lesser increase than control (24%, P<0.0001 and 16%, P<0.0001) at 75 and 100 μg mL-1). Battah et al. [11] have also reported decrease in protein content of A. variabilis with thiocarbamate herbicide thiobencarb treatment. Reports are available that protein decreases after pesticide treatments in cyanobacteria in higher concentrations [36]. The decrease in protein content may also be due to presence of pesticide beyond cyanobacteria tolerance range, higher protease activity, retarded growth, and decreases in carbon and nitrogen assimilation under stress conditions [36]. |
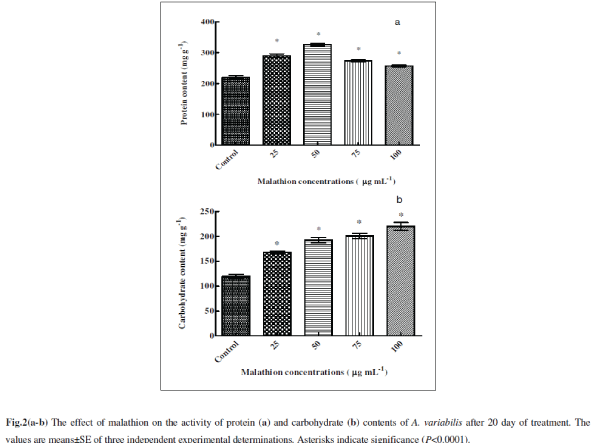 |
| The total carbohydrate content significantly increased following treatments of malathion with 40% (P<0.0001), 61% (P<0.0001), 68% (P<0.0001) and 84% (P<0.0001) respectively at 25, 50, 75 and 100 μg mL-1 compared to untreated control (Fig. 2b). A similar trend was also observed in A. variabilis and Anabaena PCC 7119 with trichlorofon (organophosphate insecticide) and thiobencarb (herbicide) treatment [11, 46] . Increase in sugar content may be an adaptive measure aimed at survival under malathion stress condition. It is generally known that when protein synthesis is suppressed by various factors, algal cells, depending on genotype, are transformed to synthesize either carbohydrates or lipids [8]. This unbalanced cell composition could be due to disturbances in nitrogen metabolism and photosynthetic activity 11. |
| C. Effect of malathion on stress parameters |
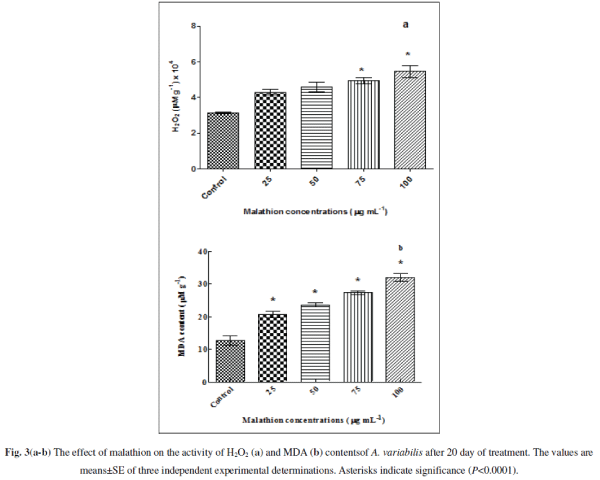
| Pesticides trigger oxidative stress by producing ROS, e.g. O2 âÂÂâ and H2O2 in cyanobacteria (Kumar et al. 2008; Fatma et al. 2007; Prasad et al. 2005). We confirmed the ROS generation by measuring the amount of H2O2 generated upon treatment of the cells by malathion with significant increment of 36.373% (P<0.01), 45.366 % (P<0.001), 56.305% (P<0.0001) and 72.894% (P<0.0001) at 25, 50, 75 and 100 μg mL-1 (Fig. 3a) respectively. The increase in the ROS generated will augment protein carbonylation, lipid peroxidations and many other alterations. Our results shown in Fig. 3b indicated that it might be most probably due to oxidative stress as the MDA level (which is a good signature of oxidative stress) increases with increasing concentrations of the pesticide. When cultures were exposed to 25, 50, 75 and 100 μg mL-1 malathion, MDA increased significantly to 63% (P<0.0001), 86% (P<0.0001), 115% (P<0.0001), and 152% (P<0.0001) respectively compared with untreated control. MDA content also increased with increasing concentrations of the organochlorine insecticide endosulfan and herbicide bentazon suggesting formation of free radicals eliciting pesticide toxicity in Nostoc. moscurum, A. variabilis, Aulosira fertilissima and Anabaena cylindrica [36, 65] |
 |
| D. Effect of malathion on enzyme activities |
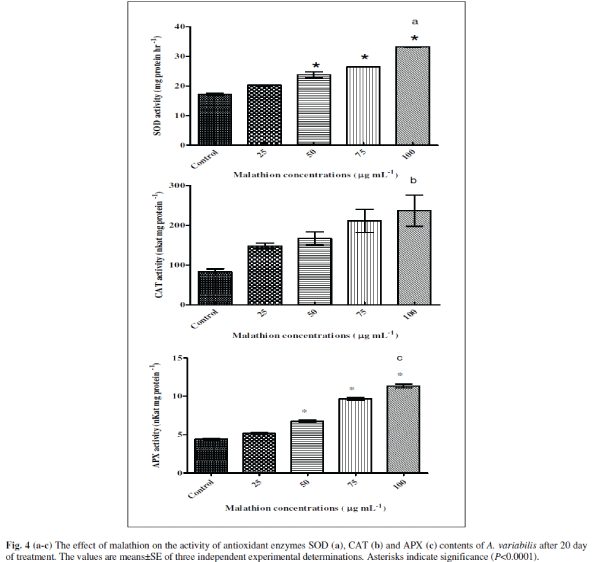
| Algae contain several enzymatic and non-enzymatic antioxidant defense systems to maintain the concentration of ROS to protect cells from damage (Noctor and Foyer 1998). The increased level of O2 âÂÂâ and H2O2 triggered the activities of several antioxidant enzymes such as SOD, CAT and APX in P. boryanum, A. fertilissima, A. variabilis and N. moscurum under test of endosulfan, organochlorine insecticide (Kumar et al. 2008 and Prasad et al. 2005). We were therefore interested in investigating strategies adopted by the cyanobacteria to circumvent the toxic effect of the malathion. Therefore, we have measured activities of three anti-oxidant enzymes, SOD, CAT and APX [Figs 4(a-c)]. Interestingly, we observed increased levels of all three of the enzyme activities suggesting the cyanobacteria try to accumulate more antioxidants so as to detoxify the various free radicals generated. The enzyme SOD was stimulated significantly with increasing concentration of malathion as 18% (P<0.001), 38% (P<0.0001), 53% (P<0.0001) and 93% (P<0.0001) at 25, 50, 75 and 100 μg mL-1 malathion respectively compared to untreated control (Fig. 4a). CAT showed 79% (P>0.01, ns), 104% (P>0.01, ns), 157% (P<0.001) and 191% (P <0.001) increase in 25, 50, 75 and 100 μg mL-1 malathion compared to untreated control (Fig. 4b). In case of APX, (Fig. 4c), 16% (P<0.01), 32% (P<0.0001), 118% (P<0.0001), and 156% (P<0.0001) were stimulated in 25, 50, 75 and 100 μg mL-1 malathion compared to untreated control in A. variabilis. A. variabilis cells counteract the toxicity of malathion induced free radical formation by increasing their antioxidant defense mechanisms. Increased levels of SOD, CAT and APX were observed in A. variabilis cultures treated with the insecticide endosulfan, suggesting that these antioxidant enzymes play a role in ROS detoxification (Kumar et al. 2008). |
 |
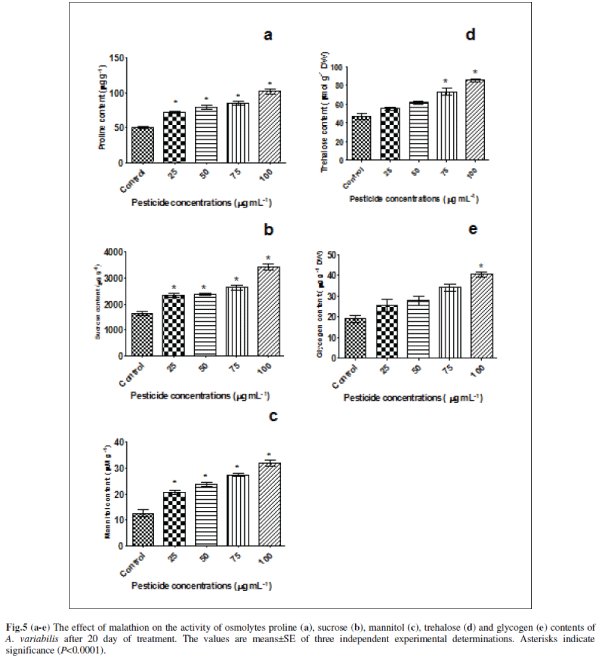
| E. Effect of malathion on osmolytes contents |
| Many plants and animals including micro-organisms accumulate small organic compounds called osmolytes to protect organisms from various stresses. We are therefore, interested to look at the levels of various endogenous osmolytes. For this we have chosen some osmolytes (proline, mannitol, trehalose, glycogen and sucrose) that are commonly accumulated by plants under various stresses (Figs. 5a-e). It was observed that all these osmolytes are induced by malathion. Proline showed a significant increase of 42% (P<0.0001), 56% (P<0.0001), 68% (P<0.0001) and 101% (P<0.0001) at concentrations of 25, 50, 75 and 100 μg mL-1 malathion compared to untreated control in A. variabilis (Fig.5a). In higher plants, in addition to its role as an osmolyte for osmotic adjustment, proline contributes to stabilizing sub-cellular structures (e.g., membranes and proteins), scavenging free radicals, and buffering cellular redox potential under stress conditions [7]. Involvement of proline in free radical scavenging has been reported for the first time from our lab in the presence of pesticides (endosulfan, alphamethrin and deltamethrin); salt stress and heavy metal in N. muscurum, A .variabilis, A. fertilissima, S. platensis-S5 and W. prolifica-Janet strain-NCCU331 [17, 24, 36]. The involvement of proline in a free radical scavenging mechanism induced by molinate herbicide was reported in N.muscorum [65]. Proline is not only one of the essential amino acids but is also an important antioxidant involved in the response to a variety of environmental stresses and suggested as a signal or regulatory molecule that can activate multiple physiological and molecular responses [7]. |
| Sucrose content also increased gradually with increasing malathion concentration as 42% (P<0.0001), 44% (P<0.0001), 61% (P<0.0001) and 110% (P<0.0001) compared to untreated control (Fig. 5b). Anabaena sp. has been reported to accumulate sucrose as their main organic osmolyte [29]. Different stress situations, which directly or indirectly cause accumulation of ROS, such as drought, salt stress, low temperature, or excess excitation energy, are associated with soluble sugar accumulation, which has generally been considered to be an adaptive response to stress conditions in higher plants [52]. Sugar metabolism and carbon skeletons are essential to the synthesis of numerous compounds that are involved in anti-oxidative protection. Glucose is also the main carbon initial precursor for carotenoid [47] and ascorbate synthesis and for carbon skeletons of amino acids, including Cys, Glu, and Gly which are the building blocks of glutathione [45. ] All of these compounds have been involved in defences against oxidative stress, through implication in ascorbate-glutathione cycles, in redox homoeostasis, and peroxide detoxification, or singlet oxygen protection [18]. |
| Mannitol increased significantly 63.16% (P<0.0001), 86.844% (P<0.0001), 115.786% (P<0.0001) and 152.631% (P<0.0001) respectively at 0.075, 0.151, 0.227 and 0.302 mM (Fig.5c). Mannitol acts as an osmoprotectant, radical scavenger, stabilizer of protein and membrane structure, and protector of photosynthesis under abiotic stress 15. The presence of mannitol in chloroplasts resulted in an increased resistance to methyl viologen induced oxidative stress in transgenic tobacco [57]. |
| In the present study trehalose also increased significantly to 17.021 % (ns), 31.206 % (P<0.001), 56.028 % (P<0.0001), and 81.56 % (P<0.0001) respectively due to 25, 50, 75 and 100 μg mL-1 malathion stress (Fig. 5d). The accumulation of trehalose is involved in responses to abiotic stress (Sakamoto et al., 2009). Trehalose accumulation during cellular stress protects cells and cellular proteins from damage by oxygen radicals [42]. In addition, trehalose is a source of carbon and energy and a signaling molecule in specific metabolic pathways [23]. |
| Glycogen content also increased to 33 % (ns), 45 % (P<0.01), 78 % (P<0.001) and 112 % (P<0.0001) respectively in 25, 50, 75 and 100 μg mL-1 malathion (Fig. 5e). However, effects of various types of stress especially on glycogen are poorly documented. Under conditions of unbalanced nutrition, cyanobacteria synthesize several types of reserve compounds, often deposited as inclusion bodies [4]. Amongst these are the carbon/energy reserve, glycogen. Glycogen is also known to be accumulated in post-exponential growth and mobilized when a nitrogen source is made available [37]. Accumulation of glycogen as a consequence of salinity stress was reported in a Scytonema species [40]. |
| These results indicate that these osmolytes compounds may have the protective effect on oxidative stress. It is well known that oxidative stress generate large amount of ROS including H2O2, superoxides and mono oxygen species. These radicals are known to interact with protein and enzyme systems damaging protein stability and function. On the other hand osmolytes are known to preferentially hydrate and therefore stabilize proteins. The effect is generally called the preferential binding effects. It is therefore expected that osmolyte increase preferential hydration while free radical denature and the combination of which leads to balancing off the effect each other thereby not affecting enzyme function and stabilizes. |
 |
CONCLUSION |
| In this investigation we showed that malathion may act on cyanobacteria by generating oxidative stress. As a result various antioxidant enzymes (SOD, CAT and APX) are induced. Also, various osmoprotectant such as proline, sucrose, mannitol, trehalose and glycogen may be induced due to the malathion stress. It is therefore possible that the antioxidant system (SOD, CAT and APX) works coordinately with the osmolyte compounds to provide relieve from the oxidative stress condition. Since osmolytes are stress protectant and are imparted to increase accumulation upon stress, the study indicates a possible role of osmolytes in circumventing malathion stress. Future studies must focus to explore the role of these osmolytes either by over express or exogenous supply. Work on these aspects is underway. |
ACKNOWLEDGEMENT |
| The authors thank the Department of Science and Technology, New Delhi, India for financial support, which enabled them to carry out the present investigation and the National Centre for Utilization and Conservation of Blue Green Algae I.A.R.I., New Delhi for providing the A. variabilis. |
References |
|