ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Taher Abdel-Aziz1, Naglaa Azab2, Nashwa M. Emara3, Mosad M. Odah4, I.M. El-deen5
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Mutations in the BRCA1 and BRCA2 genes confer greater risk of developing breast cancer. Early age at onset is generally considered an indicator of genetic susceptibility to breast cancer. We determined whether tumor pathologic features and clinical features differ in patients with and without BRCA2 mutations, the study was carried out on thirty breast cancer patients, divided as fifteen with familial breast cancer and fifteen with sporadic breast cancer. Seven cases of the familial breast cancer patients showed BRCA2 mutaion. According to this study, there was statistically significant negative correlation between frequency BRCA2 gene mutations and female age, there was negative correlation between BRCA2 gene mutations and ER receptor, PR receptor and HER2/neu status (positivity and negativity) and positive correlation between gene mutations and the pathological grade of the tumor. However, this correlation did not reach a significant level. Three of the seven BRCA2 patients weRe of triple negative molecular type (from total 5patient had triple negative type among the thirty patients). The ER positive BRCA2 patients were mainly luminal B.
Keywords |
| Breast cancer, molecular type, BRCA2 gene, hormonal receptors |
INTRODUCTION |
| Mutations in the tumor suppressor genes BRCA1and BRCA2 are believed to be responsible for the majority of hereditary breast cancer cases. It is estimated that women with BRCA1 and BRCA2 mutations have a lifetime risk of developing breast cancer as high as 87% [1].Breast cancer is a heterogeneous disease including several entities with different clinical behavior. Even tumors belonging to the same histological type can have different clinical course. Naturally, the largest group (ductal cancer) shows the highest heterogeneity. Additional information can be obtained from molecular subtyping of breast cancer and results in breast cancer classification into subgroups with different biological properties and response to treatment [2]. Despite enormous efforts to develop novel biomarkers of tumor behavior there have been few advancements beyond the use of estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2) in diagnostic practice [3]. |
MATERIALS AND METHODS |
| This prospective study was performed at biochemistry and pathology department, Benha University hospital. The study was carried out on thirty Egyptian females divided into two groups as fifteen female patients without a family history of breast &/or ovarian cancers and fifteen female patients with a family history of breast &/or ovarian cancers. Consents were taken from the subjects after being informed the aim of the study, full history was taken (Age, marital status, contraception and family history). All patients included were diagnosed to have invasive breast carcinoma. 5ml of venous blood from each subject were collected on tube containing EDTA, the sample mixed well and stored at -80 for further processing. DNA was extracted from the blood using G-spin™ Total DNA Extraction Mini Kit – (iNtRON Biotechnology, INC. - Korean Biotech Database). PCR was done for amplification of mutations in BRCA2 gene [4]. Gel electrophoresis was done for separation of DNA bands; the bands were stained with ethidium bromide [5, 6]. Formalin fixed paraffin embedded sections of breast lesions were retrieved from the department of histopathology; H&E (Haematoxylin-Eosin) stain was done, for histopathological examination typing, histologic grading [7] and TNM pathologic staging [8]. Formalin fixed paraffin embedded tissue blocks of the primary tumor tissues were evaluated for the expression of ER, PR and HER2/neu by Immunohistochemistry IHC [9, 10].Statistical pacckage for social science (SPSS) and Microsoft Office Excel were used for data processing and data analysis. For all the tests, a p value of 0.05 or less was considered for statistical significance. |
RESULTS |
| Clinical characteristics of the patient group are shown in table 1. |
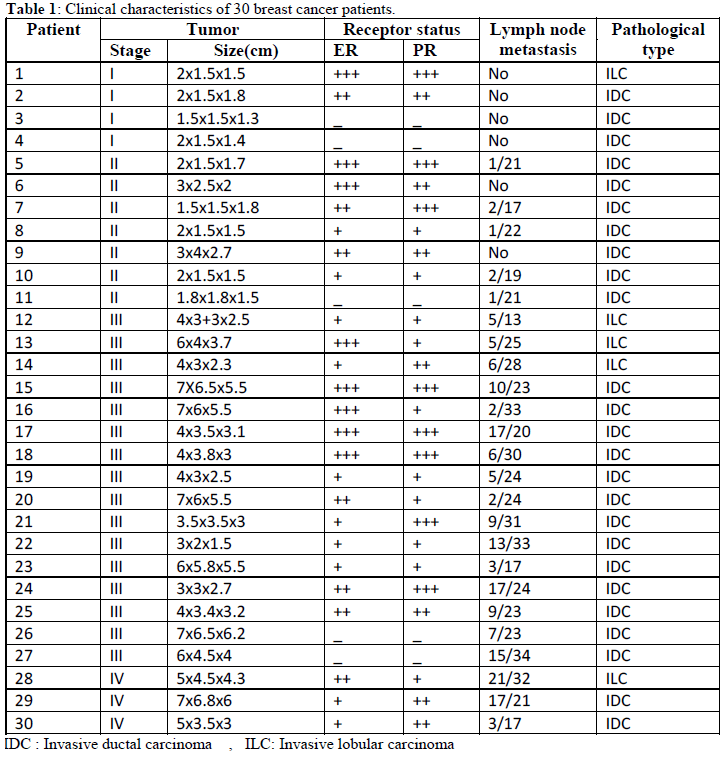 |
| The age at diagnosis of BRCA2 mutation carriers ranged from 32 to 48 years of age. The mean age at diagnosis for BRCA2 carriers was 40 years whereas for non BRCA2 carriers was 48 years. The majority of BRCA1-associated tumors were triplenegative. ER positive tumors in BRCA2-associated tumors were mainly luminal B, while for non BRCA2 tumors it was mainly of luminal A subtype. The majority of BRCA2-associated tumors were grade II/III (86%). There was statistically significant negative correlation between frequency BRCA2 gene mutations and female age in the studied groups, r = -0.42, p<0.05 (figure1). |
 |
| Figure2 shows that there was negative correlation between gene mutations and ER receptor status in patients with breast cancer groups with and without family history. However, this correlation did not reach a significant level. r = - 0.21 and p>0.05 |
 |
| Figure 3 shows that there is negative correlation between gene mutations and PR receptor status in patients with breast cancer groups with and without family history, however this correlation did not reach a significant level. r = -0.22 and p>0.05. |
 |
| Figure 4 shows that there is positive correlation between gene mutations and pathological grade of the tumor in patients with breast cancer groups with and without family history. However, this correlation did not reach a significant level. r = 0.27 and p p>0.05. |
DISCUSSION |
| In this study, we identified clinical and pathologic characteristics of tumors in women withBRCA2-positive andBRCA2- negative breast cancer. According to this study, there was statistically significant negative correlation between frequency BRCA2 gene mutations and female age in the studied groups. This also was found by Eerola et al (2005) [11]. However, studies demonstrate that BRCA2 mutations were mostly found in breast cancer patients with disease diagnosis before the age of 50 years. Moreover, in cases with familial clustering of site-specific breast cancer, BRCA2 mostly accounted for tumors diagnosed before age 40 years [12]. |
| In this study we found that there was negative correlation between BRCA2 gene mutations and ER receptor, PR receptor and HER2/neu status (positivity and negativity) and positive correlation between gene mutations and the pathological grade of the tumor in patients with breast cancer groups with and without family history. However, this correlation did not reach a significant level. |
| Previous reports describing the distribution of ER, PR, and HER-2/neu positivity in BRCA carriers have been inconclusive, mainly because in these studies, BRCA1 and BRCA2 mutation carriers were grouped together instead of being examined as two distinct groups [13]. However, some previous studies concluded that in comparison with non familial breast cancer group, the BRCA2 cancers were more receptor-negative [14]. The frequency of ERpositive cancers among our non BRCA2 cancers is quite high, which might be due to a later year of diagnosis than for BRCA2 cancers and in general because of age distribution [14]. In this study triple negative cancers were associated with presence of BRCA2 mutation and that ER positive tumors were mainly of luminal B subtype, and 86% of BRCA2 positive tumors were of II and III pathological grade, this is in accordance to studies have demonstrated that breast carcinomas in younger or premenopausal women are more likely to exhibit biologic and prognostic features that are known to be associated with a high histologic grade, such as an absence of steroid hormone receptors, It has been hypothesized that this adverse pattern of prognostic indicators and the poorer clinical outcome of younger, premenopausal patients were secondary to the greater proportion of higher grade carcinomas found in these women [14]. However, some previous studies revealed results not similar to this study as they concluded that the pathology of BRCA2-related breast cancer was similar to that of BRCA-negative breast cancers [15, 16], also researches has demonstrated that BRCA2-associated breast cancers and sporadic breast cancers are equally likely to be triple negative [17] or to have tumor pathology similar to sporadic cases [18]. One previous study suggested that BRCA2-related cancers have tumor pathology that is between that of BRCA1 and sporadic cancers [19]. |
CONCLUSION |
| We concluded from this study that BRCA2 tumors is associated with aggressive behavior and early onset tumors, further broad based studies are recommended on larger samples to identify markers indicating high possibilty of presence of gene mutations. |
References |
|