ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
M.Choukairi1, D.Bouchta2, H.Elbouhouti3, J.L. H.H. de cisneros4, I.N. Rodriguez5.
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
The objective of this work is to provide a tool for the electrochemical detection and quantification of dopamine in biological environments in the presence of ascorbic and uric acids (AA, UA). Sonogel electrodes modified with L-Cysteine prepared in this work showed very remarkable electrochemical performance for the determination of dopamine; the results obtained show good selectivity and sensitivity for dopamine, ascorbic acid and uric acid in serum. The influence of several parameters on selectivity and sensitivity were studied and optimized. The response of catalytic current with dopamine concentration shows a linear relation in the range from 10μM to 100μM with a correlation coefficient of 0, 9927 and a detection limit of 10−7 M. The applicability of the modified electrode for the analysis of biological fluid samples (serum) has been also demonstrated.
Keywords |
| Dopamine; Serum; Uric Acid; Ascorbic Acid; Sonogel-Carbon; L-cysteine. |
INTRODUCTION |
| Dopamine (DA), uric acid (UA) and ascorbic acid (AA) are compounds of great biomedical interest, playing fundamental roles in human metabolism [1]. Dopamine (DA) plays an important role in the function of central nervous, renal, hormonal and cardiovascular systems [2] It is of great clinical importance to measure the DA level in extra cellular fluid to monitor neurotransmission processes and diagnose Parkinson’s disease. There is an extensive research in the development of methods for DA quantification in blood and biological fluids. Electrochemical methods have proven to be rapid, simple and sensitive in the determination of neurotransmitters. However, an overlapping voltametric Response is usually observed because the oxidation of DA at bare electrodes occurs along with the oxidation of AA and UA in biological tissues [3–5]. AA presents in both animal and plant kingdoms, is a vital vitamin in human diet and is very popular for its antioxidant properties. It has been used for the prevention and treatment of common cold, mental illness, infertility, cancer and AIDS[6], High concentrations of molecules thus increasing the sensitivity of these materials [13-20].Nowadays there exists a great interest in the development and application of sol–gel-derived carbonbased electrodes for electrochemical applications. The sol–gel process is a chemical synthesis technique that enables the possibility of preparing a wide variety of oxide compounds at far lower temperatures than conventional methods. |
| Recently, some of us have used a new type of graphite-based sol–gel electrode, the Sonogel–Carbon electrode, which is obtained using high-energy ultrasounds. Classical procedures for the synthesis of acid catalyzed sol–gel-based electrode materials include the addition of an alcoholic solvent to the initial precursor mixture to make it homogeneous and the employment of an ultrasound bath for several minutes to promote the hydrolysis. On the contrary, by means of sonocatalysis, high-energy ultrasounds ascorbic acid, such as 34–85 and 570–3400 mol/L, can be found in human plasma and urine, respectively [7,9]. Uric acid is the major final product of purine catabolism in the human body. In a healthy human, the normal level of UA in urine is in milli molar range whereas in serum it is in micromolar range [10]. Abnormal levels of UA in the body fluids are the symptoms of many diseases like gout and Lesch–Nyhan syndrome. Electrochemical methods have been proved to be a very promising approach for the determination of AA, DA and UA due to the electro active nature of this bimolecules. But the major problem with this approach is that at conventional solid electrodes, AA, DA and UA are oxidized at potentials which are very close to each other resulting in overlapped voltammetric responses [11]. Moreover, these analyses require very high overpotential to undergo electrochemical oxidation at bare electrodes and further the electrode surface suffers fouling effect due to the accumulation of oxidation products [12]. In recent years several studies have allowed to facilitate and optimize the simultaneous detection of these three are applied directly to the precursors, and ultrasonic cavitation is achieved so that hydrol-ysis with acidic water is promoted in only a few seconds and in the absence of any additional solvent. Thanks to the phenomenon of ultrasonic cavitations, sol–gel reactions occur in a unique environment, leading to gels with special characteristics. These socalled sonogels are mainly of high density, with a fine texture and homogeneous structure. The mix of sonogel with spectroscopic grade graphite leads to the Sonogel–Carbon electrode [21,22]. The Sonogel–Carbon electrodes show the general good properties of the other CCE’s (Ceramic Carbon Electrodes). Besides, in comparison with other carbon electrodes, they exhibit especially favourable electrochemical properties, such as broad operational range of voltage and very low values of observed charging capacity (Cobs). These electrodes show very favorableelectroanalytical properties for their use as amperometric sensors and, furthermore, they easily permit the incorporation of numerous receptor molecules at the Sonogel–Carbon materials, and the deliberate chemical modification of the electrode surface with a suitable reagent results in the control of the rates and selectivity of electrochemical reactions at the solid/liquid interface [23–27]. In our laboratory The Sonogel–Carbon modified with L-cysteine was used to prepare a novel electrochemical sensor. The objective of this novel electrode modification was to seek new electrochemical performances for detection of epinephrine in the presence of uric acid. The modified electrode had also been applied to the determination of epinephrine and uric acid in biological samples with satisfactory results [28]. In another work this new electrode modification was used to seek new electrochemical performances for the detection of DA. The influence of natural interferents such as AA and UA was explored. The concentration of these strong interferents was increased to a certain level in order to determine to what extent AA and UA may disturb the neurotransmitters electroanalysis. This work shows that the modified electrode offers interesting analytical performances. Optimization of parameters such as the amount of L-cysteine in the Sonogel-Carbon mixture, interference effect, perm-selectivity and mechanical stability of the sensor are discussed. On the other hand the new Sonogel modified electrode has been applied to the determination of dopamine in urine samples with satisfactory results. The proposed sensor is a simple tool for the selective detection of DA, AA and UA in biological samples with good selectivity and sensitivity [29] . In the present work we explore the behavior of Sonogel– Carbon electrodes based on the incorporation of L-cysteine towards the electrochemical oxidation of DA and AA in serum in the presence of high concentrations of UA by different voltammetric techniques such as CV and SWV. |
MATERIALS AND METHODS |
| A. Materialsand Reagent: |
| Methyltrimethoxysilane (MTMOS) was from Merk (Darmstad, Germany), Hydrochloric acid (HCl) and sulfuric acid (H2SO4) was from Panreac (Barcelona, Spain). L-cysteine (>99%) was obtained from Fluka Chemical Company (Switzerland). UA (99%) was purchased from Sigma (Barcelona, Spain), DA and AA were purchased from Aldrich (Milwaukee, USA), and used as received. KH2PO4 and K2HPO4 for phosphate buffer were from Fluka. All reagents were of analytical grade or higher and used as received without further obtained purification. Graphite powder (spectroscopic grade RBW) was from SGL Carbon (Ringsdorff, Germany). Nanopure water was by passing twicedistilled water through a Milli-Q system (18MΩ-cm, Millipore, Bedford, MA). Glassy capillary tubes, i.d. 1.15mm, were used as the bodies for the composite electrodes. Serum group (A) rhesus negative (blood transfusion center, Tetouan, Maroc). |
| B. Apparatus |
| All electrochemical measurements were performed with an Autolab PGZ301 DYNAMIC – EIS VOLTAMMETRY – France). The experiments were carried out in a three-electrode cell at room temperature (25±1 ◦C). The counter electrode was a platinum wire and a Ag/AgCl, 3M KCl electrode was used as the reference. The composite-filled capillary tubes were used as working electrode. Square wave voltammetry (SWV) and Cyclic Voltammetry (CV) were the electrochemical techniques applied to study the behavior of the Sonogel–Carbon electrodes. Measurements were carried out under N2 atmosphere when required. The synthesis of the Sonogel- Carbon was carried out sonicating with a high-power ultrasonic generator, SONICATOR 3000, from MISONIX (MISONIX Inc, Farmingdale, NY, USA) equipped with a 13-mm titanium tip that provides a maximum power of 600W. |
| C. Preparation of the Sonogel Electrode |
| To prepare the Sonosol, the general procedure was as follows: 500μL of MTMOS and 100 μL of 0.2M HCl were mixed and then insonated during 5 s with the high-power ultrasonic processor, in this way the mixture is subjected to the phenomenon of ultrasonic cavitations, by which the sol–gel process begins, avoiding the use of alcoholic solvent and reducing drastically the time needed to get an unique phase. In the next step, the adequate amounts of L-cysteine and graphite powder were added and homogenously dispersed in the obtained Sonosol. After several minutes, the resulting material starts to acquire enough consistency thus it could fill easily the glass capillaries leaving a little extra mixture sticking out of the glass tube to facilitate the subsequent polishing step. After 24h, the Sonogel–Carbon Lcysteine composite electrode becomes hardened and, therefore, structured. Adherence between the developed material and the glass was excellent. Before use, the electrodes were polished with No. 1200 emery paper to remove extra composite material and wiped gently with weighing paper. Electrical contact was established by inserting a cooper wire into the capillary. |
RESULTS AND DISCUSSION |
| A. Dosage of Uric Acid in Serum and Validation of the Technique |
| For an initial assay of an undiluted serum sample,the measurement is performed in an electrochemical cell with a volume of 20 ml, with a potential range from -400mV to 800mV. This potential window was chosen to detect the maximum serum molecules. Fig 1-A(a) shows a peak recorded at 220mV of UA and another peak recorded at 590 mV corresponds to the molecule xanthine. Curve (b) shows the peak of the UA with a decrease in intensity due to the dilution, and a potential shift towards positive values of 60 mV due to the decrease of the pH value the disappearance of the peak corresponding to xanthine is observed because of the effect of dilution. So our study will be conducted on a sample of serum diluted 6 times for the purpose of determining the concentration of UA, and compared with the results obtained by other assays end commercial value of our electrode. After addition of different concentrations of UA in a serum sample diluted 6 times (Fig. 1 (B) curves (b at f)), peaks were obtained at the same potential than that obtained in an undiluted serum sample (Fig1 A (a)), these results confirm that this peak correspond to UA, The calibration curve shows a linear dynamic range from 2.10-5M to 10.10-5M with a correlation coeficientR² = 0,9953. The linear regression equation for this range is,Ipa (μA) = 0,943 [UA](10-5M) - 0,98(μA), using this equation Into account the dilution, the concentration of uric acid in the undiluted serum sample is 65μM. To confirm these results, we analyzed a sample of diluted serum by spectrophotometric method and we obtained a concentration the UA 78μM, this result is very close to that one we obtained with our electrochemical assay. |
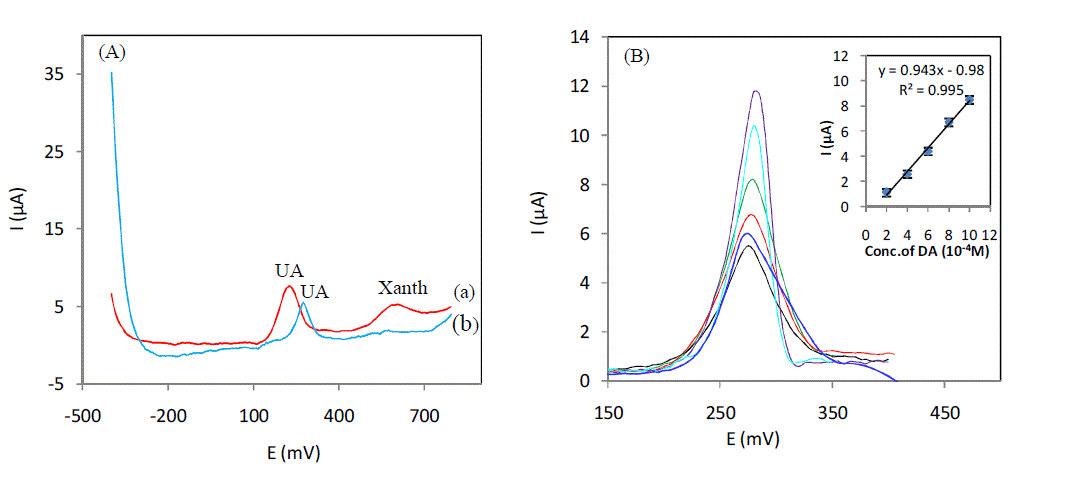 |
| B. Separation of Electrochemical Response of DA and UA in Serum |
| Curves in Fig. 2 (A) show the cyclic voltammograms of a 4μM DA and UA, at an unmodified Sonogel-Carbon (a) and a 5% L-cysteine Sonogel-Carbon modified electrode (b) respectively in serum sample. Two broad peaks were obtained in ongoing scan and convolution of the oxidation peaks has limited the selectivity and sensitivity of simultaneous determination of DA and UA at a 5% L-cysteine Sonogel-Carbon modified electrode show that modification of Sonogel-Carbon surface with L-cysteine has resolved the merged voltammetric peaks into two welldefined oxidation peaks at 185 and 350mV for DA and UA, respectively. The separation of the two oxidation peaks allows the simultaneous determination of DA and UA in a serum solution. The same catalytic behavior is observed for the SW voltammograms obtained in the serum containing the mixture of the DA and UA. Two anodic peaks at 145 and 280 mV are obtained with the modified electrode; SWV was selected as electrochemical technical for subsequent studies.Fig.3 displays the SWC obtained for the different concentrations of DA in the presence of UA in serum at 5% L-cysteine Sonogel-Carbon modified electrode. Oxidation peaks with adequate resolution for both compounds were obtained, using 5% L-cysteine Sonogel-Carbon modified electrode. The oxidation peak current of DA has built up linearly (R² = 0, 9927) with increasing concentration in the range of 10 to 100 μM, whereas the voltammetric peak current of UA remained the same. Detection limit were estimated to be 10-7M for DA in serum. |
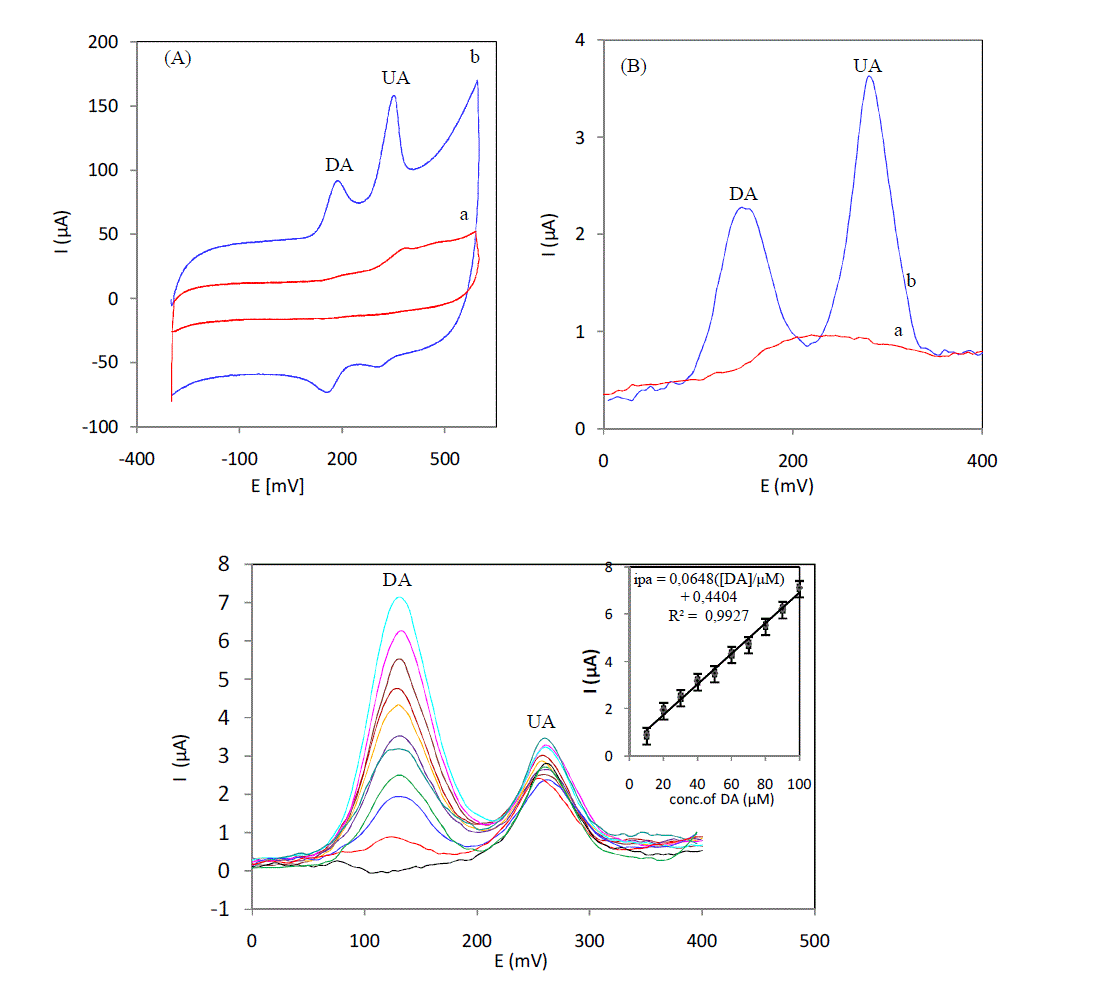 |
| C. Interferee Study of DA and AA and UA in Serum |
| AA, DA and UA coexist in serum and other extra cellular body fluids and all these bimolecular are electrochemically active. Since they have almost similar oxidation potentials at most of the conventional solid electrodes, their selective determination in a ternary mixtures is of great importance in electrochemical research. Fig. 4 (A) shows the CV curves recorded using a ternary mixture of 20μM DA,1mM AA, and 6mM UA at (a) unmodified electrode and (b) 5% L-cysteine Sonogel-Carbon modified electrode in serum sample. As isclear from the figure, at unmodified electrode, the anodic peaks of AA, DA and UA are fully merged to give a broad and overlapped peak (curve a). When 5% Lcysteine Sonogel-Carbon modified electrode was used under identical conditions, AA, DA and UA gave their oxidative peaks distinctly at –63mV, 174 mV and 350 mV respectively (curve b). The anodic peak potential separations are calculated to be 237 mV between AA and DA, 276mV between DA and UA and 413 mV between AA and UA. The electrochemical behavior of 5% L-cysteine Sonogel-Carbon modified electrode towards AA, DA and UA in a ternary mixture was further studied in SWV mode. Fig. 4B shows the SWV curves recorded using a ternary mixture of 20μM DA, 1mM AA and 6 mM AU of serum in serum sample (pH7). Both at bare and modified electrode. As it is evident from the figure, at the unmodified electrode, the oxidation peaks of AA, DA and UA are overlapped (curve a). On the other hand, the anodic peaks of the three analytes are well resolved at 5% L-cysteine Sonogel-Carbon modified electrode with peak potentials at −50 mV, 140 mV and 280 mV for AA, DA and UA respectively (curve b). The corresponding peak potential separations are 190 mV, 140 mv and 330mV for AA–DA, DA–UA and AA–UA respectively. Therefore, the extent of separations between the anodic peaks of AA, DA and UA, achieved in CV and SWV modes is large enough for the selective and simultaneous determination of DA and UA in the presence of high concentrations of AA. |
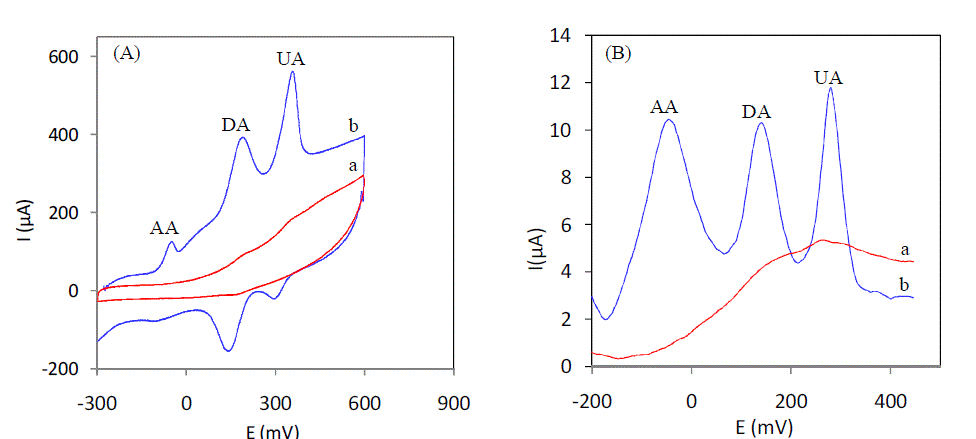 |
| D. Detection of Dopamine in Serum at Different Concentrations in Presence of AA and UA |
| The preliminary CV studies and SWV have shown that 5% L-cysteine Sonogel-Carbon modified electrode has a significant catalytic activity towards the electrochemical oxidation of AA, DA and UA compared to that at the unmodified electrode. We further investigated the electrochemical behavior of the three analytes in binary and ternary mixtures under optimized conditions in SWV mode. In the binary mixtures, they did not interfere in the detection of each other. Fig.5 shows the SWV curves for the oxidation of various concentrations of DA in presence of 1 mM AA and UA in serum sample (pH7) diluted 6 times with phosphate buffer at 5% L-Cysteine Sonogel-Carbon modified electrode. As can be seen from the graph, the anodic peak current of DA increases linearly with increasing concentration of DA in the range of 10–100μM in the presence of AA and UA. The corresponding linear function is ipa(DA)(μA) =0,4404 + 0,0648 ([DA]/μM) (R² = 0,9927). The SWV results suggest that determination of DA in presence of AA in serum could be possible as the anodic current peaks of these analyses are well separated. Furthermore, the slope of the linear regression line for the calibration graph of DA in ternary mixture is nearly equal indicating that they do not interfere with each other |
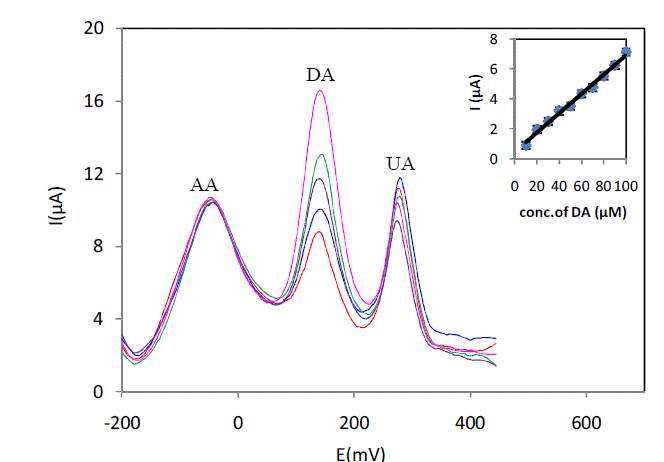 |
CONCLUSION |
| The 5% L-cysteine Sonogel-Carbon modified electrode show high electrocatalytic activity toward the oxidation of DA AA and UA in human serum and deter The 5% L-cysteine Sonogel-Carbon modified electrode show high electrocatalytic activity toward the oxidation of DA AA and UA in human serum and determination in the same medium of DA in the presence of UA and AA can be carried out with good selectivity and sensitivity. In addition, UA and AA in human serum at concentrations higher than their physiologically levels, showed no interference for DA measurement at micromolar level. Due to the attractive electrochemical and structural properties, the 5% L-cysteine Sonogel-Carbon modified electrode is expected to be a promising device for real sample biosensor applications in the biological medium. |
ACKNOWLEDGEMENT |
| This work carried out in the laboratory of Electrochimie and SystèmesInterfaciaux (ERESI), Department of Chemistry, Faculty of Sciences, University AbdelmalekEssaâdi, Tétouan, Morocco with collaboration of the Department of Analytical Chemistry Faculty of Science, University of Cádiz, Cadiz, Spain. Special regards to the laboratory analyzes ibnnafis medical, Street youssefibntachafin first floor tetouan-Morocco for chemical analysis of UA in serum. |
References |
|