ISSN: 2321-6204
ISSN: 2321-6204
1Department of Food Science and Technology, National Institute of Food Technology Entrepreneurship and Management, Kundli, Sonepat- 131028, Haryana, India.
2Department of Pomology and Post-Harvest Technology, UBKV, Pudubari, Cooch Behar, West Bengal â 736165, India.
3Department of Fruits and Orchard Management, Faculty of Horticulture, Bidhan Chandra Krishi Viswavidyalaya, Mohanpur, Nadia- 741252, West Bengal, India.
4Department of Food Engineering, National Institute of Food Technology Entrepreneurship and Management, Kundli, Sonepat- 131028, Haryana, India.
Received Date: 01/03/2016; Accepted Date: 16/06/2016; Published Date: 26/06/2016
Visit for more related articles at Research & Reviews: Journal of Food and Dairy Technology
Sohiong, Prunus nepalensis L, Anthocyanin, Antioxidant capacity, Total phenolics, Food colour, Solvent extraction.
From times immemorial, colour plays a significant role in acceptability of several products viz. textiles, cosmetics, food and other items. Colour is the main sensory attribute of food that affects the appeal and overall acceptability to the consumers. Colours are added to food owing to the losses during processing, colour replacers, enhancers, to minimize inter batch variations and also as nutrient supplements. Recently, there has been an increased interest in the development of food colourants from natural sources as alternatives to synthetic ones due to adherence to regulatory norms and health concerns [1].
Anthocyanins are the organic pigments characterized by a wide spectrum of colour tones, ranging from orange through red, to purple and blue, nearly black colours in the flowers, fruit, leaves and roots of several fruit and vegetable species. They are glycosides of polyhydroxy and polymethoxy derivatives of 2-phenylbenzopyrylium or flavylium salts [2]. The interest of anthocyanins derives not only from their colouring effect but also due to its antioxidative potential, tightness of capillary blood vessels, preventing lipid peroxidation, thrombosis aggregation, and the major detrimental factors for cardiovascular diseases [3-5]. Moreover being water-soluble its incorporation into aqueous food systems has an added advantage, which has made it as an attractive natural colourant [6]. On the other hand, there are constraints in anthocyanins usage due to their colour instability which is highly dependent on temperature, pH, presence of oxygen, light, co-pigments, metal ions, enzymes, etc [7]. As a result persistent research is going on to tap new plant sources rich in anthocyanins that exhibit stability during processing and storage [5].
The North East Region in India is considered as plethora of plant biodiversity in the world having abundant varieties of indigenous crops grown throughout the region. Although these crops have higher nutritional value than that of commercial available foods [8,9], they are highly underutilized and are just cultivated, consumed and traded locally within the states. There is an urgent need to fully commercialize and improve the utilization of such indigenous crops, being a source of natural colourant besides having potential health benefits. Sohiong (Prunus nepalensis L.) is an important indigenous plant in Meghalaya. It belongs to the family Rosaceae, also called as Khasi Cherry. The plant grows to 15-20 m height and bears fruits after seven to eight years of planting. The fruit is round in shape with smooth surface having resemblance to jamun or blackberry. This fruit with unique taste, flavour and colour is widely consumed among the local population as fresh or used for production of squash, ready to serve beverage, jams, preserves and wine. Owing to its rich reddish purple colour of the fruit, there is a probable prospective for extracting pigments to meet the growing demands of food industry and its potential applications in pharmaceutical and textile industries. Furthermore, the high content of bioactive components in fruit having strong radical scavenging activity [9] will prevent the onset and progression of degenerative diseases. The study was carried out with an avowed aim to evaluate the extraction conditions of anthocyanin enriched extracts of P. nepalensis which will boost its perspective in the food industry as a natural colourant.
Chemicals
All reagents and standards used for analysis were of analytical grade and procured from credible concerns viz. Sigma, Merck, BDH and Qualigens.
Plant samples
Ripened Sohiong fruits (P. nepalensis) were procured from a local farmer in Meghalaya, India. Fruits were washed thoroughly to remove extraneous matters. The stalk and stones were removed manually. The fruits were cut into pieces and subjected to freeze drying. The freeze dried fruit were kept below 4°C until further use. Samples were grounded to powder prior to extraction using an electric blender and passed through 0.5 mm sieve.
Determination of physicochemical properties of fresh fruit samples
The moisture content of fresh fruit samples was determined at 105°C using digital infrared moisture analyzer (Citizen MB50C) and the dry matter calculated simultaneously. Total soluble solids (TSS) was obtained by manually crushing the fruits and determined by using a digital refractometer (Atago RX-7000i) at a standardized temperature (20°C). To determine the pulp content and pulp: stone ratio of fresh fruit samples, the weight of individual fruit, pulp and seed was recorded and calculated using following equations. All readings were recorded in triplicates.
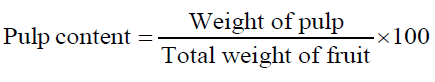
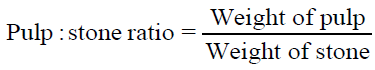
Extraction of anthocyanin from freeze dried sohiong
The grounded dried fruit samples were subjected to different extracting conditions. Optimizing various parameters during extraction may help improve extraction yield. The independent variables considered for the study were extraction time (minutes), extraction temperature (0 C), ethanol concentration in water (%, v/v) and citric acid concentration (%, w/v). Experiments were conducted using one variable at a time as given in Table 1. The powdered samples were mixed with the extracting solvent in the ratio of 1:10 in a beaker covered with aluminum foil to prevent loss of solvent. Extraction was carried out in a shaker incubator operated at desired temperature and 100 rpm facilitating mixing throughout the process. The extracts obtained from each treatment were centrifuged at 10000 rpm at 100 C for 10 minutes using refrigerated centrifuge (Sigma 3- 18 KS) and filtered using Whatman filter paper No. 1. The extracts obtained after each treatment were stored in amber coloured bottles at 40 C until analyzed. All experiments were conducted in triplicate.
| Expt. No | Variables | Levels | Constant Variables |
|---|---|---|---|
| 1 | Time (minutes) |
60, 120, 180 and 240 | Temperature: 45°C, Ethanol Conc. : 60% v/v Citric acid Concentration : 3% w/v |
| 2 | Temperature (°C) |
25, 35, 45, 55 and 65 | Time: 120 minutes, Ethanol Conc. : 60% (v/v ) Citric acid Concentration: 3% (w/v) |
| 3 | Ethanol Concentration (% v/v) |
40, 50, 60, and 70 | Time: 120 minutes, Temperature: 35°C Citric acid Concentration : 3% (w/v) |
| 4 | Citric acid Concentration (% w/w) |
0, 1, 2, 3, 4 and 5 | Time: 120 minutes, Temperature: 35°C Ethanol Concentration: 60% (v/v) |
Table 1: Experimental values for independent variables and constant variables.
Determination of total monomeric anthocyanin
The quantity of anthocyanins in extracts was determined spectrophotometrically by the pH differential method as described by Giusti and Worldstad [10]. Two dilutions of the extracts were prepared by diluting an aliquot of test samples with potassium chloride buffer (pH 1.0) and sodium acetate buffer (pH 4.5) in 10 ml volumetric flask. Appropriate dilution factor (1:100) was determined by diluting an aliquot of the test sample with potassium chloride buffer (pH 1.0) until the absorbance at 520 nm was within the linear range of the spectrophotometer (Elico SL 159 UV-Vis spectrophotometer). The absorbance was measured after 15 minutes at 520 nm and 700 nm against distilled water as blank. The monomeric anthocyanin pigment concentration (milligram cyanidin-3-glucoside equivalent /litre) was calculated using following equation:
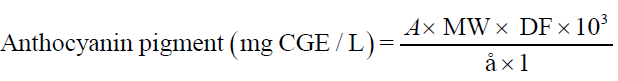
Where, A= (A520 – A700)pH 1.0 – (A520 – A700)pH 4.5; MW is the molecular weight of the pigment cyaniding-3-glucoside = 449.2 g/ mole, DF is the dilution factor = 100, 103 is the factor conversion from g to mg, ε is the molar absorptivity of cyanidine-3-glucoside = 26900 mg/L cm and l is the pathlength in cm = 1 cm.
Results were transformed and expressed in terms of milligram cyanidin-3-glucoside equivalent per 100 grams of Sohiong dry matter (mg cy-3-gl/100 g dm).
Qualitative phytochemical screening
The extract obtained from optimized extraction conditions was further subjected to qualitative screening for bioactive constituents using standard procedures as outlined by Harborne [11], Sofowara [12], and Evans [13]. The phytochemicals analyzed were saponins (foam test), phlobatanins, glycosides (Keller Killiani’s test), flavonoids (alkaline reagent test, ferric chloride test and lead acetate test), tannin and phenols (lead acetate test and ferric chloride test), amino acids (Ninhydrin test), proteins (Biuret test), carbohydrates (Fehling’s test for sugar), alkaloids (Mayer’s and Wagner’s test) and terpenoids.
Quantitative estimation
The extract obtained from optimized extraction conditions was further subjected for estimation of total phenols, flavonoids and tannin content.
Total phenol content
The total phenol content (TPC) of extracts was analyzed using Folin Ciocalteu (FC) reagent according to the protocol designed by Singleton and Rossi [14]. Briefly, 1 ml of sample was mixed with 70 ml of distilled water to which 5ml of FC reagent (diluted 1:10 with distilled water) was added. The mixture was allowed to stand for 2 minutes followed by adding 15 ml of Na2CO3 (200 g/L). The volume was made up to 100 ml with distilled water, mixed and allowed to stand for 2 hours at room temperature. The absorbance was measured at 765 nm. Gallic acid (50-500 mg/L) was used as standard and the TPC was expressed in terms of milligram gallic acid equivalent per 100 gram Sohiong dry matter (mg GAE/100 g dm).
Total flavonoids content
Estimation of total flavanoids content was carried out by colorimetric analysis using aluminium chloride assay [15]. 1 ml of diluted extract was pipette into clean test tube containing 1.5 ml methanol. 0.1 ml of 10% aluminium chloride and 0.1 ml 1 M potassium acetate solution was added and mixed thoroughly. The mixture was allowed to stand for 40 minutes at room temperature and absorbance was recorded at 415 nm using distilled water as blank. Quercetin (20-100 μg/ml) was used as standard and the results were expressed as milligram quercetin equivalent per 100 gram Sohiong dry matter (mg QE/100 g dm).
Tannin content
Tannin content was determined using Folin-Denis method. The Folin-Denis reagent was prepared by dissolving 100 g sodium tungstate and 20 g phosphomolybdic acid in 750 ml distilled water in a flask to which 50 ml phosphoric acid was added. The mixture was allowed to reflux for 2 hours and was subsequently cooled and volume made up to 1000 ml with distilled water. For total tannin estimation, 1 ml of diluted extract was added to 100 ml volumetric flask containing 75 ml distilled water. 5 ml of prepared Folin-Denis reagent and 10 ml of sodium carbonate (35 % in distilled water) was added and volume was made up to 100 ml with distilled water. The absorbance was measured at 700 nm after 30 minutes. Tannic acid (0 – 1 mg/ ml) was used as standard and the results were expressed in terms of milligram tannic acid equivalent per 100 gram Sohiong dry matter (mg TAE/100 g dm).
In vitro antioxidant activity
Antioxidant activity of the extracts obtained from identified optimal extraction conditions was assessed by measuring the radical scavenging activity of the extract using DPPH (2,2-diphenyl-1-picrylhydraxyl) assay and the reducing power of the extract using FRAP (Ferric reducing antioxidant power) assays. All measurements were conducted in three replicates.
DPPH radical scavenging assay
For DPPH assay, the method was adapted from the method given by Brand-Williams et al. [16] with minor changes. Appropriate dilution factor (1:4) was determined by diluting an aliquot of the test sample with extractant until the absorbance at 520 nm shows a reading that is within the linear range of the spectrophotometer. Briefly 0.1 ml of the diluted sample was treated with 3.9 ml of 0.1 mM methanolic DPPH solution and allowed to stand for 30 minutes in the dark at 37°C. The absorbance was recorded at 517 nm immediately against methanol as blank. Percent inhibition of the DPPH radical was calculated using the following equation:
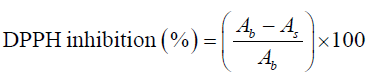
Where, As is absorbance of DPPH after reacting with given concentration of sample extract or standard and Ab is absorbance of DPPH solution with methanol blank instead of sample. The result is given as milligram Trolox equivalent antioxidant capacity per gram Sohiong dry matter (mg TEAC/ g dm) derived from standard graph using Trolox as positive standard (R2= 0.9994).
Ferric reducing antioxidant power assay
The FRAP assay was performed according to the method given by Benzie and Strain [17] with minor modifications. Sample extract was diluted 1:100 times with distilled water to obtain reading that is within the linear range of the spectrophotometer at 593 nm. 0.1 ml of the diluted sample was pipette out in a test tube with 3ml of FRAP reagent containing acetate buffer (300 mM, pH 3.6), TPTZ (0.031 mg in 10 ml 40 mM HCl) and ferric chloride (20 mM) in the ratio of 10:1:1. After 4 minutes, the absorbance was recorded at 593 nm against FRAP as blank. Result is expressed as millimol ascorbic acid equivalent per 100 gram Sohiong dry matter (mmol AAE/ 100 g dm). The FRAP value was derived from standard curve using ascorbic acid (R2 = 0.9977).
Statistical analysis
Analyses of Variance (ANOVA) were conducted by using IBM SPSS Statistcs Version 20.0 and Duncan tests were performed to test the significant differences between treatments (p ≤ 0.05).
Physico-chemical properties
The results for moisture content, dry matter, pulp content, pulp: stone ratio and total soluble solid content of fresh Sohiong fruit samples are presented in Table 2.
| Moisture content (%) | 75.8 ± 0.78 |
| Dry matter content (%) | 24.2 ± 0.51 |
| Total soluble solids (%) | 21.0 ± 0.43 |
| Pulp content (%) | 50.0-60.0 |
| Pulp: stone ratio | 1.4-2.0 |
Values Mean ± SD for three measurements.
Table 2: Physicochemical properties of fresh Sohiong (P. nepalensis) fruits.
Effect of extraction parameters on anthocyanin content
The different extraction parameters, i.e., time, temperature, ethanol concentration and citric acid in the extractant were found to have a significant effect on the extraction of anthocyanins from Sohiong (P. nepalensis). Our results showed that maximal extraction of anthocyanin was obtained under following optimized conditions, viz, 60% (v/v) ethanolic solution acidified with 3% (w/v) citric acid at 35°C for 120 minutes.
Preliminary phytochemical screening of extract
The preliminary phytochemical screening tests are useful in detection of bioactive compounds that may be present in plant extracts. As observed in Table 3, the tests revealed that most of the phytochemicals were present in the Sohiong fruit extract.
| Bioactive compounds | Acidified ethanolic extract |
|---|---|
| Alkaloids | - |
| Amino acid | - |
| Carbohydrate | + |
| Flavanoids | + |
| Glycosides | + |
| Phenols | + |
| Phlobatanin | + |
| Protein | + |
| Saponin | + |
| Tannins | + |
| Terpenoids | + |
+ = Present; - = Absent
Table 3: Phytochemical constituents of P.nepalensis anthocyanin enriched extract.
Total phenol, flavonoids, tannin contents and in vitro antioxidant activity
The total phenol content is given as gallic acid equivalent derived from standard curve using different concentration of gallic acid (R2=0.9992) whereas the total flavonoid content was derived from standard curve using quercetin (R2=0.9982) and total tannin is expressed as tannic acid equivalent (R2= 0.9990). The Sohiong anthocyanin extract obtained from the optimized extraction condition was estimated to contain high total phenol (812.8 ± 3.42 mg GAE/100 g dm), flavonoids (449.4 ± 3.85 mg QE/100 g dm) and tannin content (143.6 ± 0.06 mg TAE/100 g dm). The DPPH and FRAP assays also revealed that the extract exhibited very strong antioxidant activity (16.6 ± 0.07 mg TAEC/g dm and 31.9 ± 0.14 mmol AEAC/100 g dm respectively) as shown in Table 4. The comparative DPPH inhibition capacity of trolox, BHT, ascorbic acid and Sohiong extract are presented in Table 5.
| Total Phenolic Content (mg GAE/100 g dm) |
812.8 ÃÂ ± ÃÂ 3.42 |
| Total Flavonoid Content (mg QE/ 100 g dm) |
449.4 ÃÂ ± 3.85 |
| Total Tannin content (mgÃÂ TAE/100 g dm) |
143.6 ÃÂ ± ÃÂ 0.06 |
| DPPH scavenging capacity (mg TEAC/ g dm) |
16.6 ÃÂ ± ÃÂ 0.07 |
| FRAP (mmol AEAC/100g dm) |
31.9 ÃÂ ± ÃÂ 0.14 |
Values Mean ± SD for three measurements.
Table 4: Quantitative estimation of total phenol, flavonoid, tannin and Invitroantioxidant activity Sohiong (P. nepalensis) anthocyanin enriched extract.
| Sample | DPPH Assay | |
|---|---|---|
| Concentration | DPPH Radical Scavenging activity (%) | |
| Trolox | 50 μg/ml 100 μg/ml 200 μg/ml 300 μg/ml |
12.61 ÃÂ ± ÃÂ 0.32 26.11 ÃÂ ± ÃÂ 0.55 49.42 ÃÂ ± ÃÂ 1.00 73.15 ÃÂ ± ÃÂ 1.00 |
| BHT | 50 μg/ml 100 μg/ml 200 μg/ml 300 μg/ml |
13.91 ÃÂ ± ÃÂ 0.06 20.34 ÃÂ ± ÃÂ 0.41 37.81 ÃÂ ± ÃÂ 0.22 56.55 ÃÂ ± ÃÂ 0.42 |
| Ascorbic acid | 50 μg/ml 100 μg/ml 200 μg/ml 300 μg/ml |
18.44 ÃÂ ± ÃÂ 0.41 31.31 ÃÂ ± ÃÂ 0.69 56.18 ÃÂ ± ÃÂ 0.98 87.07 ÃÂ ± ÃÂ 0.68 |
| Sohiong anthocyanin extract | 5 μl /ml 10 μl/ml 15 μl/ml 20 μl/ml |
34.66 ÃÂ ± ÃÂ 0.15 58.49 ÃÂ ± ÃÂ 0.58 75.15 ÃÂ ± ÃÂ 0.84 81.42 ÃÂ ± ÃÂ 0.48 |
Values are Mean ± SD for three measurements.
Table 5: Percentage DPPH radical scavenging activity of Sohiong (P. nepalensis) extract and Trolox, BHT and ascorbic acid.
Physical chemical properties such as moisture content, dry matter, total soluble solids and pulp content are important fruit quality attributes. Moisture content determination in food is considered as one of the most important assays since moisture greatly influences the physical properties and stability of the food [18]. The dry matter and total solids are also important characteristics as these parameters highly determine the taste and processing of the fruit [19]. Total soluble solid (T.S.S.) is one of the quality traits in a fruit which also influences the taste and act as an indicator of maturity of the fruit [20]. The results obtained in the study for physico-chemical analysis of the fresh fruit samples of Sohiong are in full agreement with those reported in earlier studies by Patel et al. [21] and Rymbai et al. [22].
Anthocyanins are bioactive compounds in plants that have been extensively studied due to the many possible health benefits [23] and the extraction of these compounds from several plant sources have been conducted over the recent years. The efficiency of an extraction process greatly depends on various input parameters as well as understanding the nature of the plant matrix. Extraction variables such as type of size particle of solid, type of solvent, solid: solvent ratio, time, temperature, etc. have been reported to have an effect on the extraction yield and quality of the final extract to be used either in the food or pharmaceutical industries [24]. The study also showed the effect of the extraction parameters, viz., time, temperature and concentration of ethanol and citric acid on the extraction of anthocyanin from freeze dried Sohiong. Initially it was observed that increasing the extraction time from 60 to 120 minutes resulted in a significant increase in anthocyanin content in the extract indicating that less time may not be sufficient for the solvent to penetrate deep enough into the particles to extract the required anthocyanins. However, on increasing the extraction time to 180 minutes, a non significant (p ≥ 0.05) decrease in the anthocyanin content was observed. Furthermore, on increasing the extraction time to 240 minutes resulted in a significant decrease (p ≤ 0.05) of anthocyanin content in the extract. The results are in conformity with those found by Thao et al. [25] who reported a reduction in amount of anthocyanins in purple rice extracts on increasing extraction time from 150 to 180 minutes. Escalating the extraction time may have caused oxidation of anthocyanins compounds that resulted in degradation and loss of anthocyanins in the extracts. From economic viewpoint, 120 minutes was chosen as the optimal time for extraction of anthocyanin from Sohiong. This result was used for subsequent experiments.
Further, the effect of temperature on extraction of anthocyanin from dried Sohiong fruits was investigated. Temperature has a great impact on the physical properties of the extracting solvent, such as viscosity and surface tension, which influences the diffusion and rate of transfer of solutes through the plant cells into the solvent [26]. Increasing the temperature during extraction improves the mass transfer rate and solubility of anthocyanins into the solvent, but very high temperature treatments have been reported to cause degradation of anthocyanins which results in lowered anthocyanin content in the extract [27]. The effect of temperature on extraction of anthocyanins from the Sohiong freeze dried samples. On increasing the extraction temperature from 25°C to 35°C, the total anthocyanins content increased from 767.0 ± 10.25 mg cyd-3-glu equivalent/100g dm to 837.7 ± 8.38 mg cyd-3-glu equivalent/100g dm with subsequent decrease at higher temperatures (p ≤ 0.05). Similar studies have been observed in previous study conducted by Cacace and Mazza [28].
The selection of type of solvent and its concentration can significantly affect the extraction of anthocyanins from plant components [29]. Numerous solvents have been employed to extract anthocyanins from plants for many years and methanol has been reported to be the most efficient and commonly used for extracting anthocyanins from plant sources [30]. But in the study, extraction was carried out with acidified ethanol solution at various concentrations in water, considering it is for food applications and its cost effectiveness. Varying the ethanol concentration in the extractant causes variation in the physical properties, like boiling point, viscosity and density, thus modifying the polarity which in turn greatly influences the extraction of anthocyanins from the substrate [31]. The effect of increase in ethanol concentration total anthocyanin content. The highest anthocyanin content was observed at 60% (v/v) ethanol concentration. An increasing trend was observed in anthocyanin content upon increasing the ethanol concentration up to 60% (v/v) which henceforth decreased with further increase in ethanol concentration.
Anthocyanins are reported to be highly unstable in neutral or basic solutions and therefore are commonly extracted with acidified solvents [32]. Although several researchers have employed solvents slightly acidified with strong acids such as hydrochloric acid during extraction of anthocyanins. However, too strong acid may cause hydrolysis or degradation of acylated anthocyanins. To avoid or minimize the breakdown of acylated anthocyanins, organic acids are generally preferred over strong acids [33]. In the present study, citric acid was the choice as it has multifold applications in food and can be easily eliminated during anthocyanin concentration. The variation in the total anthocyanin content corresponding to citric acid concentration revealed that the maximal level of anthocyanin extraction was achieved at 3% (w/v) acidification. Further increase in citric acid concentration concomitantly decreased the anthocyanin content. This result showed that citric acid concentration of >2% and <4% would be best to achieve high recovery of anthocyanin during extraction.
Our study reveals maximal extraction of anthocyanin (837.74 ± 8.38 mg cyd-3-glu equivalent/100 g dm) from Sohiong fruit using 60% (v/v) ethanolic solution acidified with 3 % (w/v) citric acid at 35°C for 120 minutes. From our results it was observed that Sohiong fruits possess total anthocyanin content which is at par with other commercial fruits like Rubus idaeus (Raspberry, red) and Aronia melanocarpa (Chokeberry, black) and much superior to Fragaria ananassa (Strawberry) and Vitis spp. (Grape) which have been reported to contain (171-1030) mg/ 100 dm, 177-1052 mg/ 100 g DM, 97-520 mg/100 dm and 113 mg/ 100 g dm respectively [34].
The extract obtained from above optimized operating conditions was further subjected for phytochemical screening and for estimation of total phenol, total flavanoids, total tannin content and in vitro antioxidant activity. Preliminary phytochemical screening revealed the presence of bioactive compounds attributing to its antioxidative potential. Studies have confirmed that these phytochemicals largely accounts to the therapeutic properties and antimicrobial activity in several medicinal plants, viz., terpenoids have been reported to have anticarcinogenic, anti-ulcer, antimalarial, hepaticidal, antimicrobial and diuretic activities. Likewise, phlobatanins have also been observed to have diuretic properties [35]. Saponins have been reported to have cardioprotective properties and glycosides are known for treatment of congestive heart failure and cardiac arrhythmia [36]. Furthermore, phenols, flavonoids and tannins are largely known to contribute antioxidant activity to plant foods. Earlier studies have shown that there is an inverse association between intake of phenols, flavonoids and tannins and occurrence of cardiovascular diseases. These compounds even have a protective effect against cancer [37,38].
The anthocyanin extract obtained from Sohiong showed high content of total phenols, flavonoids and tannin reflecting its excellent antioxidant profile and associated health benefits. The antioxidant potential of Sohiong extract was evaluated by two methods, the DPPH scavenging assay and ferric reducing antioxidant power (FRAP) assay. It is always desireable to perform different methods to determine the antioxidant activity of plant extracts because of the chemical complexicity and diversity of phenolics present in plant species [23]. Moreover, Xu et al. [39] too noted that the antioxidant effect of a substance may be accounted to different mechanisms such as reducing properties as well as free radical scavenging capacity, decomposition of peroxides or prevention of chain initiation. The Sohiong extract exhibited high antioxidant activity with DPPH radical scavenging capacity of 16.61 ± 0.07 mg TEAC/ g dm and FRAP value of 31.87 ± 0.14 mmol AEAC/100 g dm. Our results indicate that Sohiong has a potent antioxidative potential which is comparatively higher as compared to other fruits commonly consumed like apples, mango, papaya, plums and pomegranate arils. The FRAP value for apples, mango, papaya, plums and pomegranate arils are 1.86 – 6.07, 0.58 – 2.82, 0.14, 3.24 and 7.28 mM Fe (II)/ 100 g dm respectively [40]. With its high antioxidant capacity, Sohiong fruit can be helpful for prevention of several chronic diseases. This possibly may enhance the potential interest on this underutilized fruit and thus may improve its production, commercialization and utlization in value added products and for various applications in food and pharmaceutical industries.
The study demonstrated that the variables, viz., time, temperature, ethanol and citric acid concentraion of solvent greatly influences the total content and quality of anthocyanins from Sohiong fruit (Prunus nepalensis). It was demonstarted that maximal extraction of anthocyanin was achieved by employing ethanol solution 60% (v/v) acidified with 3% (w/v) citric acid at 350C for 120 minutes. Sohiong fruit is a good resource for anthocyanins which can be used as natural colourant for the food sector. The Sohiong anthocyanin enriched extract possess high antioxidative potential due to the presence of bioactive compounds implying it possible usage in pharmaceutical sector as well. With the increasing demand for healthy foods having natural antioxidants, there is great opportunity to enhance the production, commercialization and utilization of such high valued indigenous fruits. Further research needs to be carried out to evaluate the stability of the Sohiong extracts and the effect of incorporation of the anthocyanin extract in different food products.
We would like to express our sincere thanks to Ms. Flora Swer and Mr. H. Talang for providing the raw materials and to Dr. Ajit Kumar, Vice Chancellor, NIFTEM, for facilitating the research work and for providing technical support.