ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Y.Suresh1, S.Annapurna2, A.K.Singh3, G.Bhikshamaiah4
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Copper nanoparticles were prepared via a simple green chemical reduction method. This method was proved to be an efficient method for the preparation of copper nanoparticles at around room temperature without using any inert atmosphere. The synthesized copper nanoparticles were characterized by using Scanning Electron Microscopy (SEM), Transmission Electron Microscopy (TEM), X-Ray Diffraction (XRD), UV-Visible Spectroscopy and Fourier Transform Infrared Spectroscopy (FTIR) experimental methods. The resulted copper nanoparticles were FCC crystalline with an average particle size 5 nm. The UV absorption peak found at around 578 nm was assigned to the absorption of copper nanoparticles. The FTIR spectra showed that the thin layer of tea decoction molecule was developed on the surface of copper nanoparticle that protects it from oxidation for about 25 days.
Keywords |
| copper nanoparticle, green synthesis, chemical reduction, tea decoction, no inert gas protection, FTIR |
INTRODUCTION |
| Noble metal nanoparticles found many applications in different fields such as catalysis, photonics and electronics, because of their unique optical, electronic, mechanical, magnetic, and chemical properties. As metal nanoparticles are widely used in the areas of human contact, the necessity to develop eco-friendly methods for nanoparticle synthesis that do not use toxic chemicals has been constantly growing. Green Synthesis of copper nanoparticles is of great interest because of many advantages. Copper is highly conductive and also cheaper than silver and gold. However, aggregation and oxidation are the main problems concerned to the copper nanoparticles. But, we can overcome these problems by selecting suitable stabilizer for capping of copper nanoparticles. Several synthesis methods such as metal vapour deposition, electrochemical reduction, thermal decomposition, chemical reduction etc. have been proposed to synthesize nanoparticles. Chemical reduction is the highly preferred method among all other methods as it is simple and economical. The chemical reduction methods using separate reducing and stabilizing agents are known to generate copper nanoparticles with controlled size and shape. The chemical reduction of metal salt in aqueous solution and precipitation of the nanoparticles is a novel approach. It was reported that the two stage chemical reduction process is necessary to avoid the formation of copper oxide [1-6]. |
| Decoction method involves mashing followed by boiling in water to extract desired materials. This can be used to make teas, coffees and similar other solutions. Tea decoction is the name of the liquid resulted from boiling of tea powder in water. Present work is concerned to the rapid synthesis of copper nanoparticles via green chemical reduction route using new natural stabilizer tea decoction. Useful chemical strategies based on this synthesis have been established. This method allowed the environmentally friendly reaction conditions to obtain copper nanoparticles [7-10]. |
II. LTERATURE SURVEY |
| Maintaining product integrity is the main challenge to the preparation of nanoparticles via green approach. In 2000, Chen and Murray et al. has reported the preparation of hexanethiol protected gold nanoparticles. The green synthesis of nanoparticles using natural extracts is a new approach. The synthesis of metal nanoparticles using natural extracts is greener from a chemical hazard standpoint and advantageous from an economical point of view (Huang et al., 2007). The green reduction of metal nanoparticles using biomolecules present in plant extracts such as enzymes, proteins, amino acids, vitamins, polysaccharides has been deeplystudied in recent years (Iravani, 2011). The ecofriendly and rapid synthesis of silver, gold and copper nanoparticles using broth extracts of different plants has been reported, such as Aloe Vera (Chandran et al., 2006), Medicago sativa (Gardea-Torresday et al., 2002), Pelargonium graveolens (Shiv Shankar et al., 2003), Azadirachta indica (Shiv Shankar et al., 2004), Avena sativa(Armendariz et al., 2004), wheat (Armendariz et al., 2004), Tamarindus indica(Ankamwar et al., 2005), lemongrass (Shiv Shankar et al., 2005), Emblica officinalis(Ankamwar et al., 2005), Humulus lupulus (Rai et al., 2006), Spinacia oleraceaand Lactuca sativa(Kanchana et al., 2011) and Capsicum annum (Jha and Prasad, 2011). Jha and Prasad (2011) addressed that such studies could prove to have an enormous impact in the immediate future if plant tissue culture and downstream processing procedures are applied in order to synthesize metallic as well as oxide nanoparticles on an industrial scale. |
III. EXPERIMENTAL |
| The raw materials used for the preparation of copper nanoparticles were copper sulphate, tea decoction, L-Ascorbic acid, NaOH and Hydrazine Hydrate (HH). All chemicals used were of analytical reagent grade. The solutions were made with Millipore water. |
A) Synthesis of copper nanoparticles |
| In the present study, copper nanoparticles were synthesized by using a simple chemical reduction method. In this method, Copper sulphate was used as basic precursor, tea decoction as stabilizer, HH as reducing agent and L-Ascorbic acid as an anti-oxidant agent. NaOH was used as catalyst and also to adjust the PH to 12. Tea decoction (10%-20%) was separately prepared. The Copper sulphate (0.04M) and L-Ascorbic acid (0.001M) solutions were prepared separately using Millipore water. The solutions of tea decoction and L-Ascorbic acid were added to copper sulphate solution under rapid stirring. Then the solutions of HH (1M) and NaOH (0.01M) were added to the mixed copper salt solution under stirring. The initial blue color of the reaction mixture eventually turned to brown-black color. Stirring was continued for another 1 hr. to complete the reaction. The precipitate was washed twice with methanol after filtration and then dried to obtain copper nanoparticles. The formation of copper nanoparticles was explained as following. First the Cu2+ cations were reduced to Cu0 atoms and then Cu atoms form clusters. The tea decoction solution was used to prevent Cu cluster from aggregation through the ion-dipole intermolecular forces. This results into the formation of Cu nanocrystals. |
B) Characterization |
| The morphology and size of the copper nanoparticles were investigated using Scanning Electron Microscope (SEMHitachi S-3400N equipment) and Transmission Electron Microscope (EM-Phillips equipment). The XRD patterns of copper nanoparticles were recorded using Philips X-ray diffractometer coupled with graphite monochrometer. The scanning was done in the Bragg‟s angle region (2θ) from 10° to 80° at a speed of 0.25° per minute using Cu Kα radiation. The Crystallite size of the copper nanoparticles was calculated using Williamson – Hall equation given as following. |
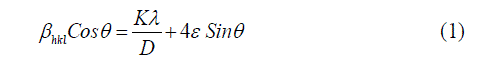 |
| Here is the wavelength of X-rays, βhkl is the full width at half maximum of X-ray profile, D is the diameter or crystallite size of the particle, ε is the lattice strain and θ is the Bragg angle. The UV-Vis spectrometer (Lab India Instruments Pvt. Ltd, Lab India UV- 3000+) was used to study the surface plasmon peak of colloidal dispersion of copper nanoparticles. All spectra were corrected against the background spectrum of water as reference. The FTIR spectra of copper nanoparticles were recorded by KBr pellet method using FTIR spectrometer (Bruker Optics, Germany, Tensor 27). |
IV. RESULTS AND DISCUSSION |
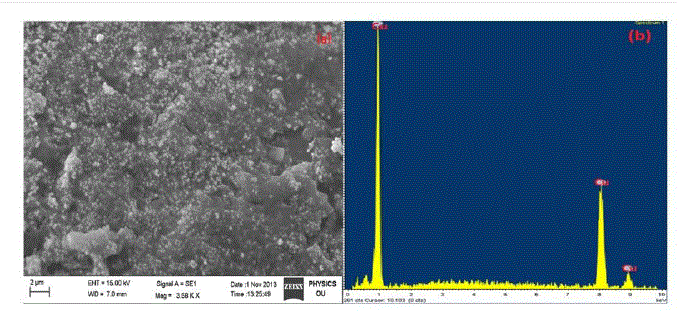 |
| The SEM image of tea decoction stabilized copper nanoparticles prepared from copper sulphate salt is shown in Fig. 1(a). From the image, clearly the copper nanoparticles formed are of spherical morphology with an average particle size around 5 nm. The composition oftea decoction stabilized nanoparticles prepared from copper sulphate salt is further probed by Energy-Dispersive X-ray (EDX) analysis and is shown in Fig. 1(b) which indicates the presence of Cu phase only. |
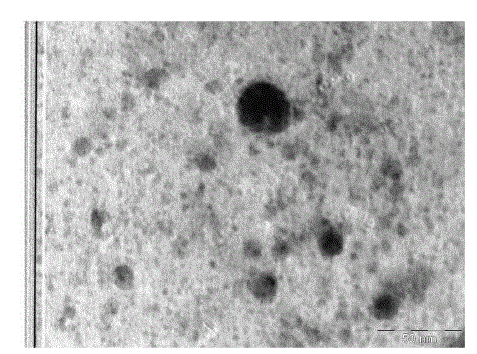 |
| TEM was employed to characterize the size, shape and morphology of prepared copper nanoparticles. The TEM image of tea decoctionstabilized copper nanoparticles synthesized from copper sulphate is shown in Fig. 2. From the TEM image clearly the morphology of copper nanoparticles is spherical. The average size of copper nanoparticles is found to be around 5 nm which is in good agreement with the particles size calculated form SEM results. |
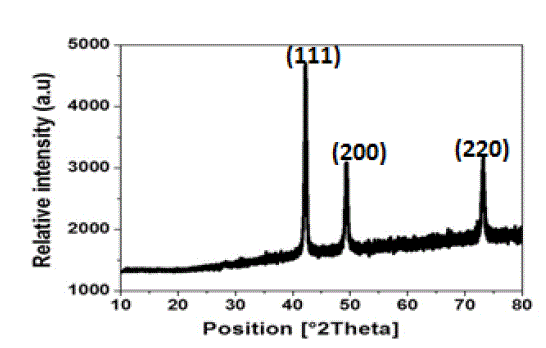 |
| The X-ray diffraction pattern of tea decoction stabilized copper nanoparticles prepared from copper sulphate is shown in Fig. 3. Three main characteristic diffraction peaks for Cu were observed at around 2θ = 43°, 50°, 74° correspond to (111), (200), (220) crystallographic planes of face-centred cubic (FCC) Cu crystals (JCPDS No.04-0784). The lattice parameter „a‟ has been calculated by using these profiles and the average value of lattice parameter is found to be in agreement with reported value 3.615 Å in literature (11). The crystallite size of copper nanoparticles is calculated using equation (1) and found to be around 3 nm which is in good agreement with the particle size measured from SEM and TEM images. |
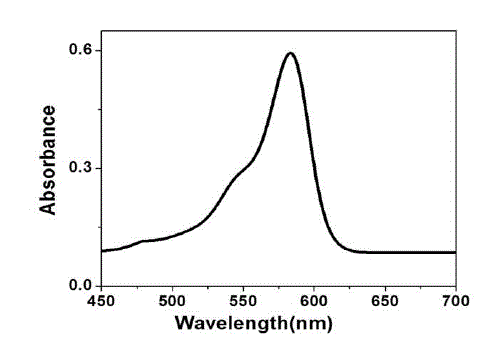 |
| The UV-Visible absorption spectrum of tea decoction stabilized copper nanoparticles prepared from copper sulphate is shown in Fig. 4. The copper nanoparticles prepared have displayed an absorption peak at around 578 nm. This peak can be assigned to the absorption of copper nanoparticles. This spectrum confirms the presence of copper only, as there is no other measurable peak observed. |
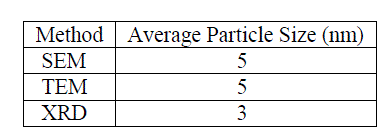 |
| Table 1 report the summary of mean particle sizes determined from SEM, TEM and XRD experimental methods. From the summary, it appears that the apparent, morphologically spherical copper nanoparticle size is on the order of nanometers. This is consistent with previous analyses of the powders (12). |
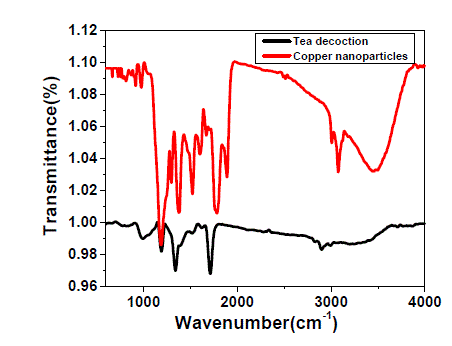 |
| The FTIR spectrum of pure tea decoction and copper nanoparticles stabilized by tea decoction are shown in Fig. 5. The broad and strong bands are observed at around 3470 cm-1 and 628 cm-1 corresponding to the O-H stretching frequency. For pure tea decoction, the peaks in the range of 650-1000 cm-1 are attributed to the C-H bending vibrations of –HCCH- links of tea decoction molecules. Also, the band observed at around 981 cm-1 was attributed to the C-O stretching, which is the characteristic of either functional moiety. It is clear from the Fig. 5 that these peaks in the range 650-1000 cm-1 are shifted slightly to higher wave numbers in the FTIR spectrum of tea decoction stabilized copper nanoparticles. These differences indicate that the thin layer of tea decoction molecule is developed on the surface of the copper nanoparticle. |
| The tea decoction stabilized copper nanoparticles prepared are found to be stable in air for about 25 days and have shown no contamination during this period. However, the copper nanoparticles washed in hot water have shown contamination with copper oxide after few days. This confirms that the tea decoctionmolecules on the surface of copper nanoparticles are important to avoid oxidation. Because, when washed in hot water the tea decoction stabilizer layer on the surface of copper nanoparticle will be removed. The prepared tea decoction stabilized copper nanoparticles are useful in medical area. |
V. CONCLUSIONS |
| The green synthesis of tea decoction stabilized copper nanoparticles at around room temperature has been reported. The copper nanoparticles prepared are stable in air with respect to oxidation for about 25 days; because of the thin layer of tea decoction molecules surrounded the nanoparticle. The average particle size is found to be around 5 nm. |
ACKNOWLEDGEMENTS |
| The authors thank Director, DMRL, Hyderabad and CFRD (Central facilities for Research and Development), Osmania University, Hyderabad for providing experimental facilities. The authors extend thanks to Dr.B.Sreedhar (IICT, Hyderabad) for giving valuable suggestions and extending TEM facility. The authors are also grateful to the Head, Department of Physics, Osmania University, Hyderabad for the needful assistance. |