ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
B.V.Rao*, A.D.P.Rao, V.Raghavendra Reddy
|
| Corresponding Author: B.V.Rao, |
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
To understand the impact of Mo6+ on some structural parameters of copper ferrites, it was substituted in the chemical compositional formulae Cu 1.0-3y Fe 2.0-2x Mo x + y O4.0. Spectra of FTIR and Mössbauer were recorded at room temperature. These are found to show three principal bands of absorption in the range of lower x or y values and started to show a shoulder or split at higher values of x and or y values in the FTIR studies. Based on the Mössbauer spectra, Magnetic hyperfine field, Isomer shift and Line width values are evaluated. Thus generalized chemical compositional formula has been proposed for cation distribution in the Mössbauer studies. The obtained results and evaluated values are interpreted on the basis of different possible mechanisms
Keywords |
| Copper ferrite, Concentration, Molybdenum, FTIR, Mössbauer |
INTRODUCTION |
| Copper ferrite is known to have its own identity and importance over all other spinel ferrites because of its existence in two crystallographic lattice structures namely tetragonal and cubic besides its phase transition at certain critical temperature and concentration of Cu2+ where as other spinel ferrites show only cubic structure. The temperature of the order – disorder transformation depends on the content of octahedral cupric ions and on the non stoichiometry [1]. Its spinel lattice is highly distorted (c/a ~ 1.06) because of Cu2+ ion, as it is a Jahn-Teller(JT) ion arising from the octahedral cupric ions [2,3] and also shows the inability to have a cation/oxygen ratio higher than 3/4. However, on the other hand it found to show anomalous favourable properties [4, 5] for different applications. Part of the Cu2+ ions can be frozen in tetrahedral sites when the ferrites were quenched in air from above the 400°C [6]. The resulting ferrite material shows smaller tetragonal distortion since a great proportion of the cupric ions exist on tetrahedral sites. This sort of behaviour is assumed to show impact on the position and valence of the ions in the crystal structure and Infrared spectra can give information about position and valence of the ions in crystal lattices. Mazen et al. [7] have observed two main absorption bands 1 2 , for Cu-Ti ferrite at 1 = (540 ± 5) cm-1 and 2 = (385 ± 5) cm-1 reflecting these bands as common feature of ferrite. According to Waldron‘s classification [8], vibrations of unit cell of a cubic spinel can be constructed from the contributions of tetrahedral site (A-site) and octahedral site (B-site) atomic vibrations. So the absorption b and 1 arises by the stretching vibration of the tetrahedral metal oxygen band, 2 occurs due to octahedral metal-oxygen band. IR spectral study on Cu-Ti mixed ferrite by Elfaki et al. [9] revealed twoabsorption bands at 575 cm-1 and 400 cm-1. Cation distribution of a ferrite was proposed earlier from various studies viz. X-ray diffraction and Mössbauer effect [10]. Using magnetization [11] and Curie temperature [12] studies also distribution of cations was evaluated. Cervinka et al. [10] studied the distribution of copper ions by means of X-ray diffraction and saturation magnetization measurements in some Cu-Mn ferrites. The amounts of copper in octahedral |
 |
SAMPLES PREPARATION |
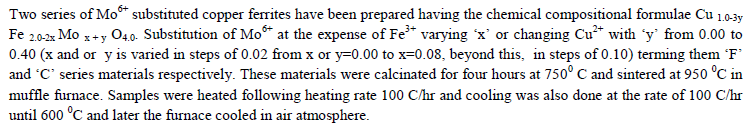 |
EXPERIMENTAL DETAILS |
 |
RESULTS AND DISCUSSION |
| The obtained spectra of FTIR and Mössbauer studies have been analysed and interpreted the results as given below. |
| A FTIR Studies |
 |
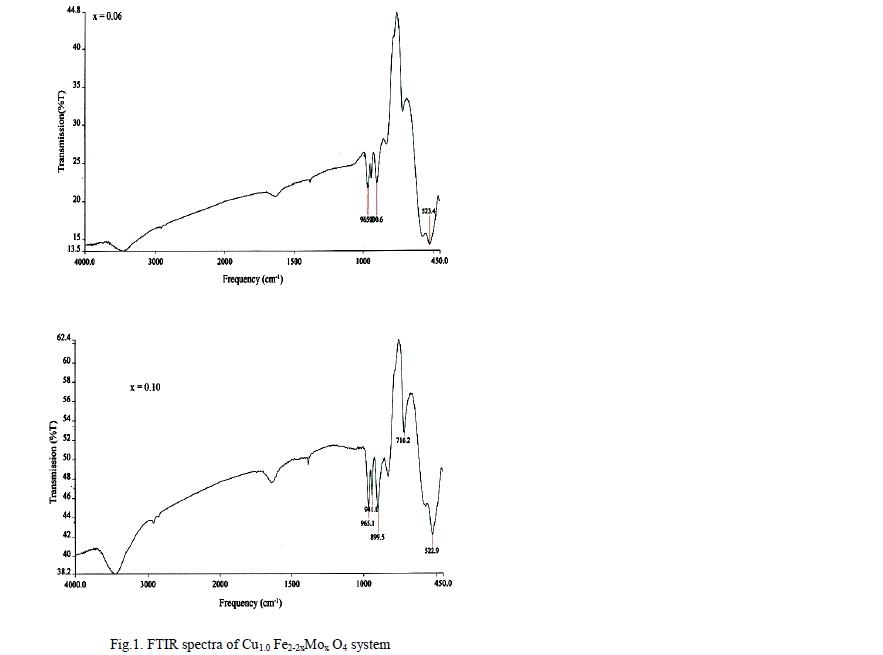 |
 |
 |
 |
 |
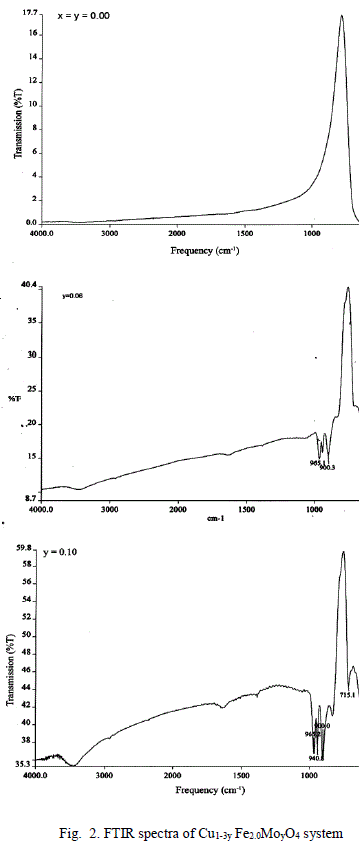 |
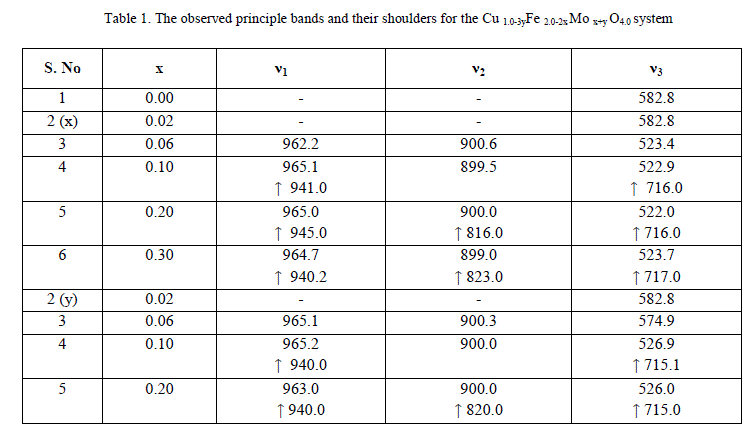
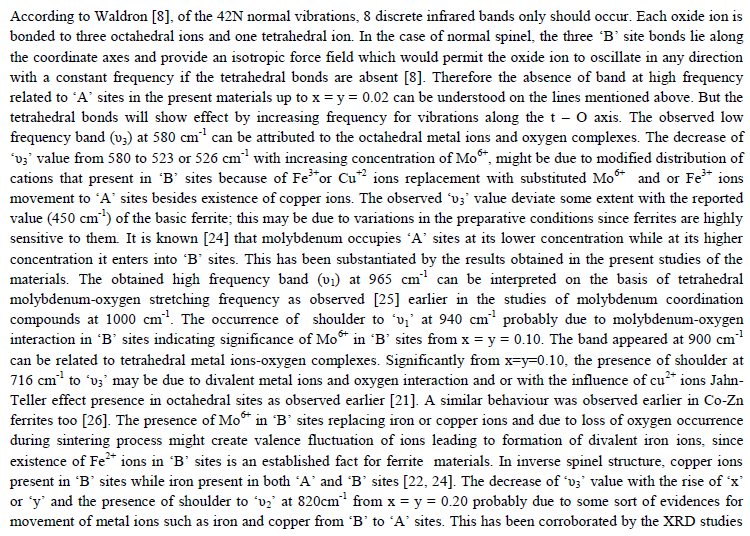
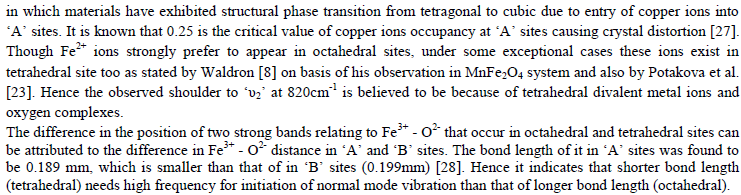
| The values of bond lengths have been computed and found the decrease of these values with âÂÂx‘ and or âÂÂy‘ indicating increased closer association of metal ions and oxygen due to substituted impurity. Hence increase of frequency value for normal mode vibration of these sites is expected, contrary to this, decrease of these values in the present studies is observed. This could be accounted by a substantial role of crystal field effect [29] that accounted by copper ions. No infrared studies on Cu-Mo-Fe system are available in literature and thus proper data is not existed for comparison of the obtained results. Around 3445 cm-1 a band has been observed for both the series of ferrites from x = y = 0.06 onwards, which may be related to the electronic transitions as studied earlier by Waldron [8] for Ni and Zn ferrites which exhibited at 2700 and 2300 cm-1 respectively. However, according to him, these values are subject to considerable uncertainty, because the portion of scattering caused by the ferrite particles cannot be distinguished from absorption losses. |
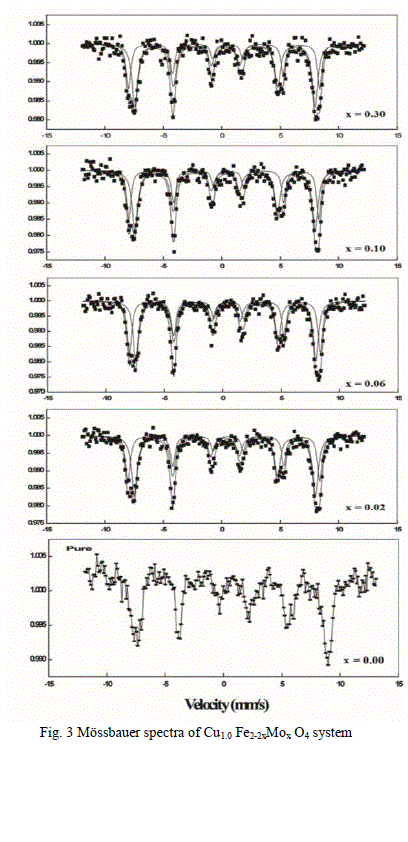
| B Mössbauer studies |
 |
 |
 |
 |
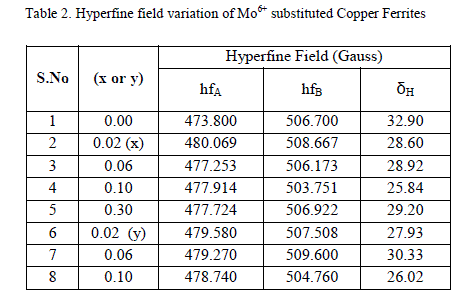 |
| Based on the obtained magnetization, Curie temperature and other experimental results in association with the Mössbauer studies, the cation distribution for both the series has been proposed. The chemical compositional formula of the present copper ferrite with the substitution of Mo6+ is given below |
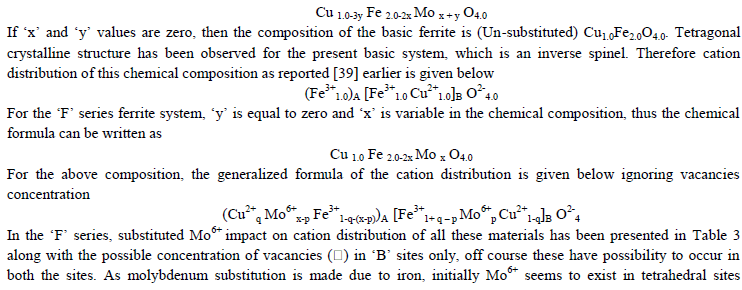 |
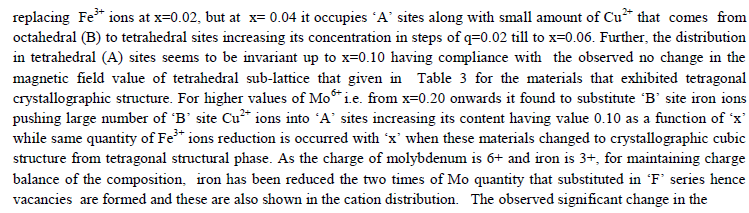 |
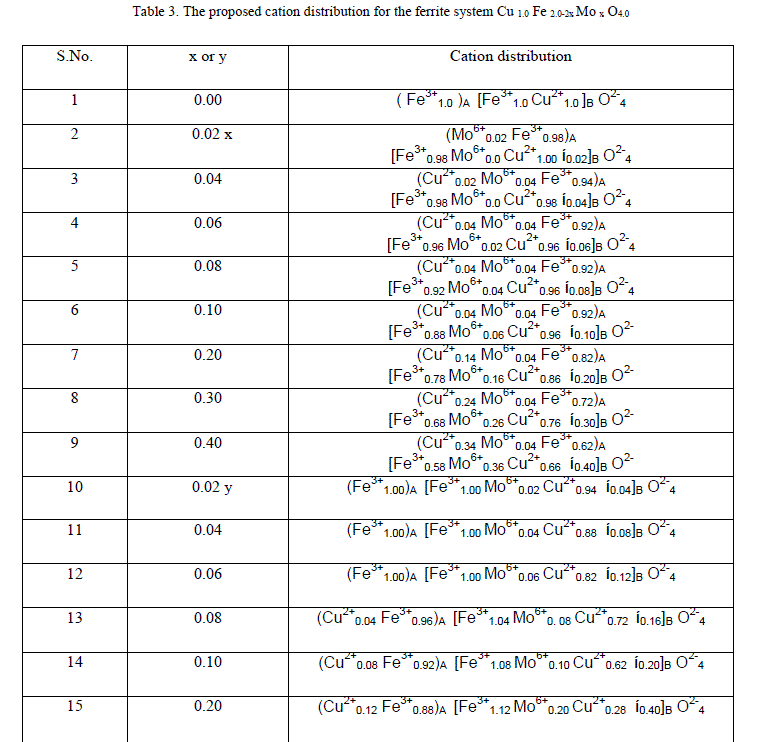 |
 |
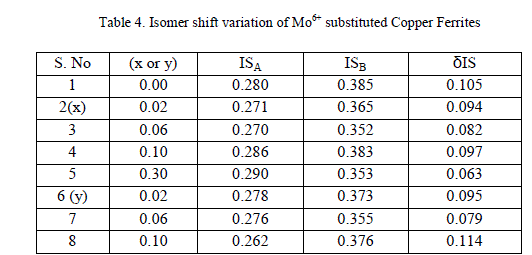 |
 |
 |
CONCLUSIONS |
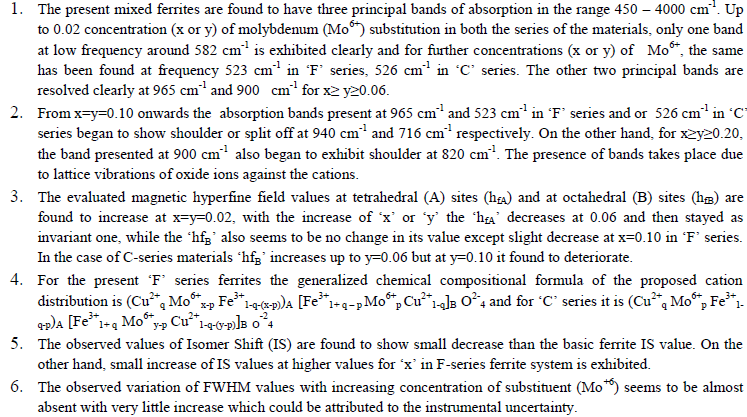 |
ACKNOWLEDGEMENTS |
| One of the authors B.V.Rao, is grateful to the UGC, New-Delhi for providing teacher fellowship to carry out this work. |
References |
|