ISSN ONLINE(2319-8753)PRINT(2347-6710)
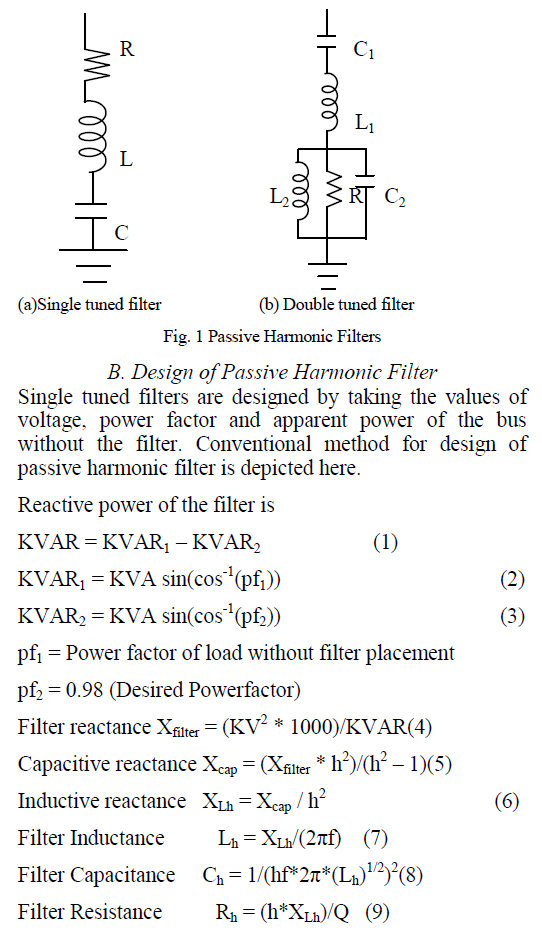
ISSN ONLINE(2319-8753)PRINT(2347-6710)
K S Sivaraman1, Dr S Shanmuga Priya2
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Activated Carbon prepared from Sugarcane Bagasse(SC) was selected as the suitable adsorbent for CO2 sequestration and an experimental analysis was carried out to study the effectiveness of adsorption of the vehicular exhaust on the adsorbent. The adsorbent was prepared by slow pyrolysis of Sugarcane Bagasse at 600oC. Because of the increasing level of greenhouse gases in the atmosphere due to our dependence on fossil fuels, the need to create a cost efficient,reusable material with a high CO2 adsorption capacityhas become necessity. In this paper the FTIR analysis of the SC600oC was carried out. The vehicular exhaust was injected into a thin film reactor after H2S removal. The effectiveness of adsorption was tested at an emission testing centre while removal of H2S was tested by simple qualitative analysis.
Keywords |
| CO2 sequestration, Low-cost adsorbent, Sugarcane Bagasse, Vehicular Exhaust, Thin Film Reactor |
INTRODUCTION |
| Large scale development of modern civilization has led to very fast depletion of fossil fuel [15].The usage of fossil fuels is a major aspectleading to the increase in the concentration of CO2. It has been predicted that fossil fuels will continue to be heavily used around the world for many years because of increase in the demand for energy [8].The recent scientific research shows that there is a direct correlation between global warming and concentration of greenhouse gases. The percentage increase in the concentration of CO2which was 1% per year has increased to >2% over the last 25 years [12]. IPCC estimates that the average CO2 concentration may rise to 570 ppm by the year 2100 resulting in the increase in the global temperature and the rise in the sea level [15]. Not only selecting alternative energy source is important, but, controlling CO2 is equally important for relieving the greenhouse effect [19]. Atmospheric CO2is reversible and if the emissions are reduced enough then the oceans will take up the anthropogenic CO2[1]. CCS has emerged as a viable option to reduce CO2emissions, which uses low carbon energy [2]. The major drawback of CCS is the high energy demandcaused by the CO2.separation from gases [16]. CO2 sequestration can be done by three approaches: 1. Pre-combustion process; 2. Post combustion process; 3. Oxy-fuel combustion process. Among these post combustion method has the greatest potential for reducing CO2levels, as these processes can be retrofitted to existing units, thus providing a quicker and economical way to reduce the cost for CO2sequestration [14]. Novel methods can be used for this purpose which can make the operation cost effective. These methods includeenzyme based techniques, absorption, membrane separation, adsorption, bio-fixation [4]. Amine has been commercially used as a solvent for absorption,but it suffers from high regeneration cost, equipment corrosion, and amine degradation due to oxidation [20]. Very little has been found out about in situ algal cultivation using flue gas [10]. Adsorption has been considered the most viable and economic solution. The two types of adsorbents are natural and activated. The natural adsorbents are made using living systems or biomass, whereas the activated adsorbents are produced using high carbon percentage and minimum inorganic compound containing materials. A lot of research is being carried out to produce low cost adsorbent from cheaply available agricultural waste, as they are an excellent and economical source for producing activated carbon because of the low ash content and reasonable solidity present in them. Some of these sources are: Sugarcane Bagasse, Hazelnut shell, etc. Because Van Der Waal force i.e. physical adsorption plays a major part in sequestering CO2, the adsorbent can be reused by regenerating using chemical, pressure or temperature swing operations.The vehicle exhaust consists of CO, CO2, Hydrocarbons, Particulate Matter, Nitrous oxides, H2Sand Lead, which needs to be reduced to protect the environment. |
| The excess CO2if utilized properly can become a blessing as it can lead to better alternatives for the present fuel demand b [18]. It will open a new sector for energy which was previously not accessible. If the carbon dioxide is recycled using renewable energy, this could provide us with an economical way of delivering energy to the consumer [3]. |
MATERIALS AND METHODS |
Materials |
| a) Activated Carbon prepared using pyrolysis of Sugarcane Bagasse at 600oC |
| b) Bellow for capturing vehicular exhaust |
| c) Steel sheets |
| d) Zinc Oxide |
| e) Syringe |
Methods |
| In the present research the method of Adsorption using SC600oC has been used, because of its high regeneration ability and low cost. |
FTIR |
 |
| FTIR spectrophotometer (Fig 1) was used to characterize the SC600oC. First the sample was prepared by mixing the sample and KBr (10:1 ratio) in a mortar and a thin transparent pellet was then made by applying hydrostatic pressure using the lever manually. TheFTIR was then openedand the sample pellet was then kept on the mount for the analysis. The plotbetween Transmittance v/s Wavelength was obtained was then interpreted. The various steps mentioned above have been depicted in Fig 2. |
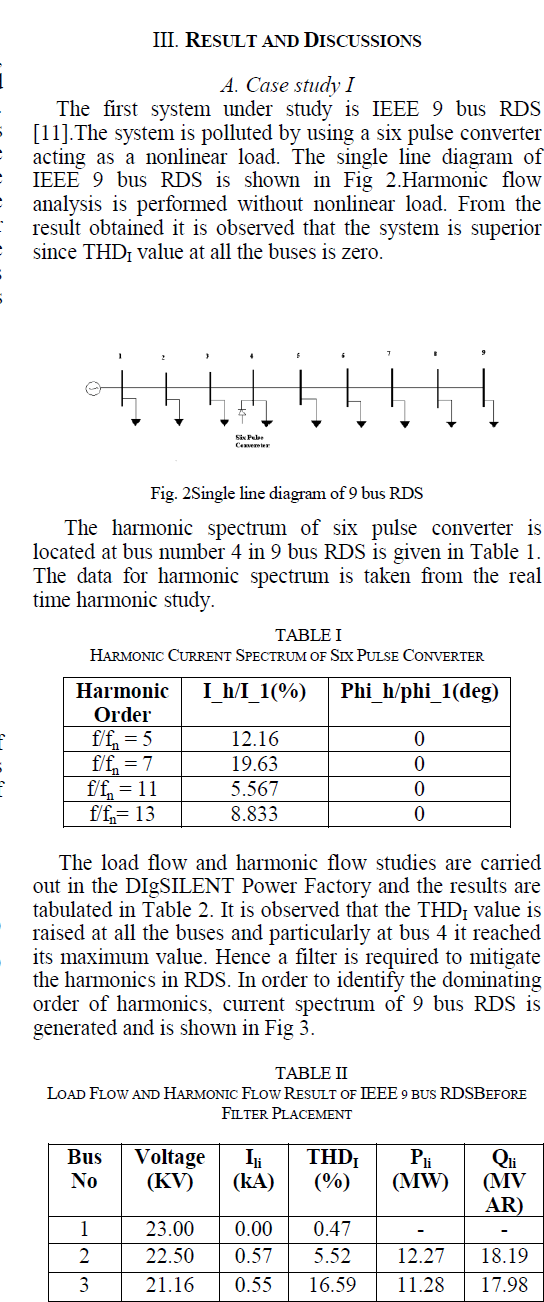 |
| The above figure (Fig 2) gives a better idea about the different steps that were followed to characterize SC600. |
Fabrication of the reactors |
| Three reactors were used in this research two of which were thin film reactors fabricated from steel of the dimensions 30 x 23 x 2 cm. One of these reactors was used for CO2adsorption while the other was used for H2S removal. |
| The third reactor was a continuously stirred slurry reactor which was fabricated using a 1L glass beaker, rubber lid for the top and a stirrer. This reactor was also used for H2S removal. |
H2S Removal |
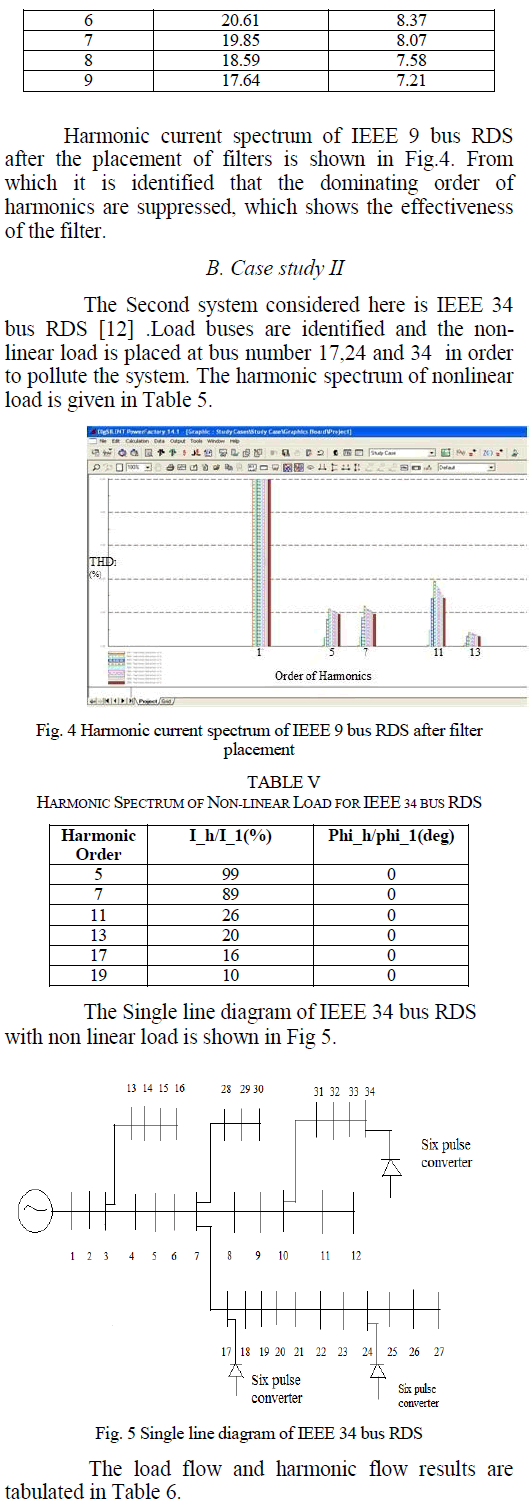 |
| ZnO was used to remove the H2S present in the exhaust gas since H2S reacts with ZnO to produce ZnS and H2O. Two different types of reactors were used to check the removal of H2S namely; a) Thin film bubbling reactor b) Continuously stirred slurry reactor, shown in Fig 3a) and 3b) respectively. Fig 3 also shows the reaction happening between ZnO and H2S present in the exhaust. |
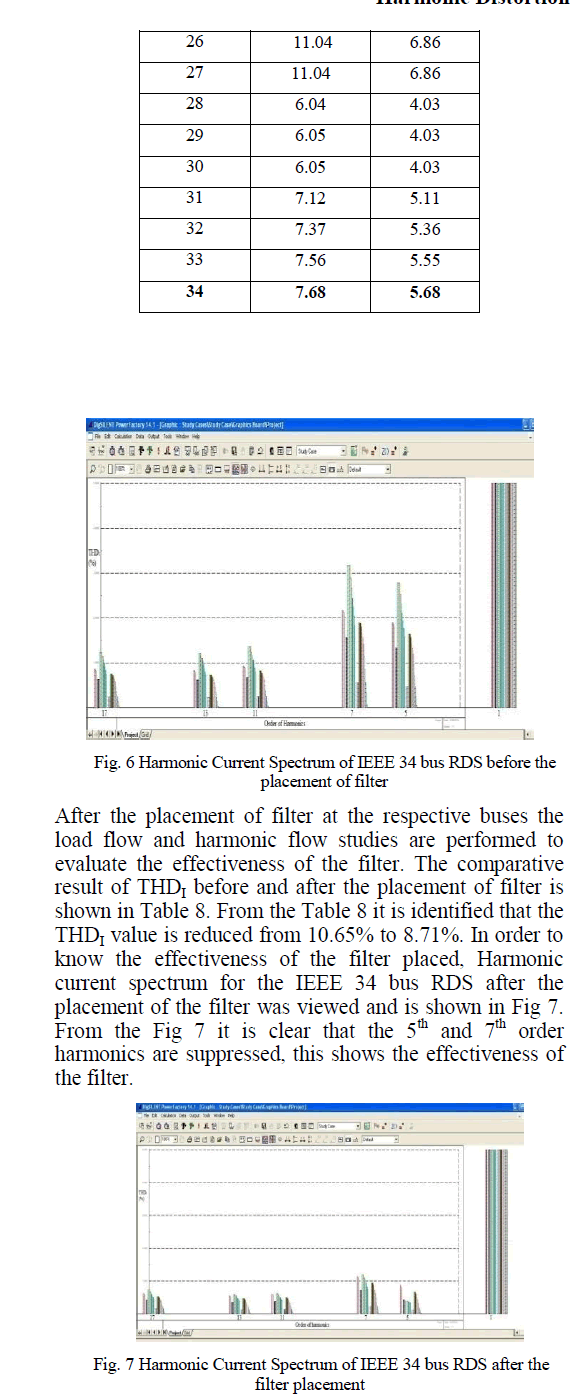 |
| Fig 4 shows the porous tube and thin film reactor without the reactants to show the arrangement of the porous tube inside the reactor. 8.25 g/l of ZnO slurry was used in both the reactors. The thin film bubbling reactor was equipped with a porous tube, while the continuously stirred slurry reactor used an electric stirrer. The effectiveness of conversion for both the reactors were validated using simple qualitative analysis of the salt product. The final reaction product (ZnS) from both the reactors were divided into 10 equal parts (by weight), i.e. 10 parts for each reactor. Each part was tested for the presence of Zn2+ and S2-using the group identification (Group IV and dilute HCl/H2SO4 group respectively) and confirmatory tests. And the probability of conversion was calculated for each reactor. |
CO2Sequestration |
| SC600oC was uniformly spread in the thin film reactor and sealed from the top. The vehicular exhaust was then introduced into the reactor and the inlet and outlet tubes were closed using a pinch cork. The experiment was carried for the reactor temperatures ranging from 40oC to 100oC and the temperature for the adsorption was attained using a hot air oven. The coolant used for the reactor was ambient air. The inlet concentration of pollutant for all the different temperatures was kept constant as shown in Table 1. |
 |
| From the above table we see that the concentration of NOx is zero because of negligible oxides of nitrogen being present in the vehicular exhaust. The reactor was kept undisturbed for 3 hrs. After the adsorption is carried out the unadsorbed gas was collected in a syringe (100ml) and immediately tested for emission concentrations in an automobile emission testing centre. The SC600oC was regenerated using Temperature Swing Adsorption at high temperature in an oven to desorb the adsorbed gases after experimentation of each reactor bed temperature. Fig 5 shows the various steps involved in the sequestration of CO2. |
 |
| The above figure (Fig 5) also clearly shows the thin film reactor that was used for the sequestration, the bellow in which the gas was collected and the Gas Analyser used to check the composition of the unadsorbed gas. |
EXPERIMENTAL RESULTS |
FTIR |
 |
| The plot obtained from the FTIR of SC600oC is shown in Fig 6. From the figure and from the standard FTIR tables we see thatin the functional group region (2000-4000 cm-1) alcohols and phenols (3733.93 cm-1) and Nitriles (2227.63- 2360.71 cm-1) are present. While, in the fingerprint region (500-2000 cm-1) presence of unsaturated Aldehydes and Ketones (1701.10 cm-1), Saturated Aliphatics (1739.67 cm-1), Amines (987.49 cm-1), and carbon nitrogen bonds was seen. These might be present because of the partially burnt hydrocarbons present in the exhaust gas. The presence of electron rich and electron deficient pairs along with the other N2 groups in SC600oC helps in the better adsorption of the exhaust gas which leads to a better reduction in emission concentration. |
H2S Removal |
| From the qualitative analysis of the 10 samples for each of the reactors, it was found that the continuously stirred slurry reactor showed a better performance in comparison to the thin film bubbling reactor. The above result was inferred because of the higher probability of the sample to show the tests for both S2- and Zn2+ions for the continuously stirred slurry reactor which had the probability of 0.8, whereas, the thin film bubbling reactor had a probability for a successful reaction of only 0.4. |
CO2 Sequestration |
 |
| From the results shown in Table 2 it can be concluded that the physisorption of CO2 onto the SC600oC increases with increase in reactor bed temperature, even though the increase was not very high. But, for bed temperatures above 80oC (in this case 90oC and 100oC) in the present reactor used, the decrease in the adsorption capacity/potency of the SC600oC was observed, which may be because of the desorption of the adsorbed gases at high temperatures. |
CONCLUSION |
| This work shows that activated carbon produced from agricultural waste has a good CO2sequestration ability. After checking the performance of the adsorbent in reducing the vehicular emission we can conclude that the performance increases with increase in temperature but it should not be increased too much also as it may lead to the degradation of the material. The FTIR helped us in identifying the factors that helped in the better adsorption of the exhaust gases and also gave us an idea about the functional groups present in SC600oC. H2S removal was necessary as H2S reduces the adsorption potency of the Activated carbon. Thus, if utilized properly then the problem of global warming and pollution can be efficiently reduced. |
References |
|