ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Jayashri N.Nair1, Ruthvik Nitin.P2
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Bio-Diesel produced from Waste vegetable oil(WVO), straight vegetable oil (SVO) or animal fat is as effective as Petroleum diesel.This study deals with preparation of Bio-Diesel by Transesterification of Sunflower oil (triglycerides) with Methanol and NaOH as catalyst at specific temperature resulting in formation of methyl-esters (Biodiesel). The by-product, glycerol wasseparated and the methyl-ester was washed and dried. The produced biodiesel was tested for its calorific value(38,048 KJ/Kg). The engine performance was studied with 100% biodiesel (B100) and Diesel on a single cylinder, 4 stroke compression ignition engine in terms of Mechanical Efficiency, Break Thermal Efficiency and Specific Fuel Consumption. These parameters were calculated for different loads andcompared for both the fuels. The results showed that both the fuels were very close and similar, however the specific fuel consumption for B100 was slightly higher than the Diesel.
Keywords |
| Biodiesel; Transesterification; Sunflower oil; Methyl esters; Engine performance. |
I. INTRODUCTION |
| The world is now completely dependent on burning the fossil fuels including coal, oil and natural gas, which are currently the world's primary energy source.[1]. The depletion of oil reserves and increased environmental concerns has laid the interest in using bio-fuels for running engines and in power generation. Biodiesel is renewable, biodegradable, oxygenated and environmental friendly bio-fuel with which can deliver performance very similar to petroleum diesel. Biodiesel is closely similar to diesel with respect to viscosity, density and surface tension. There is no requirement of altering the design of an engine or no adjustment is required to operate it on bio-diesel. The cloud point (CP) which is the temperature at which the fuel forms solid phase and Cold filter plugging point (CFPP) which is lowest temperature at which the biodiesel can pass through a standard filter at specific conditions, is higher than that of diesel fuel and this problem can be solved by blending biodiesel with low sulphur diesel. Biodiesel, technically known as mono-alkyl ester of long chain fatty acids is derived from vegetable oils or animal fats by process called Transesterification by adding methanol [2]. Transesterification can be done using three types of catalysts: alkali catalyst, acid catalyst and lipase as catalyst [3]. Using a strong alkali catalyst for transesterification is most widely used and economic process because of the reasons such as high conversion rates (98%), minimum reaction time, low temperature and pressure. Strong alkali such as Sodium hydroxide (NaOH) or Potassium hydroxide (KOH) is generally used. The amount of alkali catalyst (Noah) to be used for this process may range from 0.5% to 1% w/w [4]. The amount of Methanol to be used is derived from the experiments performed by Bernard Freedman [5], where a molar ratio of 6:1 is very much suitable and can obtain 98% conversion of vegetable oil into biodiesel. The standard temperature for the reaction to occur when using an alkali catalyst is 600C to 700C [3]. In this study the sunflower oil is converted into Biodiesel and is tested for the calorific value. The biodiesel is tested for its performance, on a single cylinder diesel engine test rig operating at different loads. The results are then compared with the performance parameters of same engine operated on diesel. |
II.PREPARATION OF BIO-DIESEL |
| The world is now completely dependent on burning the fossil fuels including coal, oil and natural gas, which are currently the world's primary energy source.[1]. The depletion of oil reserves and increased environmental concerns has laid the interest in using bio-fuels for running engines and in power generation. Biodiesel is renewable, biodegradable, oxygenated and environmental friendly bio-fuel with which can deliver performance very similar to petroleum diesel. Biodiesel is closely similar to diesel with respect to viscosity, density and surface tension. There is no requirement of altering the design of an engine or no adjustment is required to operate it on bio-diesel. The cloud point (CP) which is the temperature at which the fuel forms solid phase and Cold filter plugging point (CFPP) which is lowest temperature at which the biodiesel can pass through a standard filter at specific conditions, is higher than that of diesel fuel and this problem can be solved by blending biodiesel with low sulphur diesel. Biodiesel, technically known as mono-alkyl ester of long chain fatty acids is derived from vegetable oils or animal fats by process called Transesterification by adding methanol [2]. Transesterification can be done using three types of catalysts: alkali catalyst, acid catalyst and lipase as catalyst [3]. Using a strong alkali catalyst for transesterification is most widely used and economic process because of the reasons such as high conversion rates (98%), minimum reaction time, low temperature and pressure. Strong alkali such as Sodium hydroxide (NaOH) or Potassium hydroxide (KOH) is generally used. The amount of alkali catalyst (Noah) to be used for this process may range from 0.5% to 1% w/w [4]. The amount of Methanol to be used is derived from the experiments performed by Bernard Freedman [5], where a molar ratio of 6:1 is very much suitable and can obtain 98% conversion of vegetable oil into biodiesel. The standard temperature for the reaction to occur when using an alkali catalyst is 600C to 700C [3]. In this study the sunflower oil is converted into Biodiesel and is tested for the calorific value. The biodiesel is tested for its performance, on a single cylinder diesel engine test rig operating at different loads. The results are then compared with the performance parameters of same engine operated on diesel. |
II.PREPARATION OF BIO-DIESEL |
| For the preparation, fresh virgin sunflower oil was used which was transesterified using NaOH(catalyst). Sunflower oil was first heated in a steel utensil up to 600C and the temperature was maintained constant. 3.5 grams of lye (NaOH) per litre of oil was measured accurately and dissolved completely in 200 ml of methanol (CH3OH) per litre of oil, resulting in Methoxide solution. This solution was dissolved in the preheated sunflower oil and the whole mixture was mixed/stirred thoroughly using an electrical mixer at 400 rpm for about 40 minutes. Immediately after mixing, glycerol separation was seen. The mixture was allowed to settle for about 48 hours after which the bio-diesel (methyl ester) layer at the top and the by-product glycerol layer at thebottom respectively. Glycerol was carefully separated by gravity method. The biodiesel was washed gently using warm distilled water for about 3 times. The washed Bio-diesel was dried in the open air for 3 days so as to remove excess methanol and water. The density of the bio-diesel was found to be 0.866 gm/cc at 25 0C. The gross calorific value of the bio-diesel have been tested at a certified Laboratory [7] by IS 1448 (P-6) test method which resulted as to 38,048 KJ/Kg. The properties met the minimum requirement for B100 blend based on ASTM D 6751 (B100) standards. Fig.1 a) shows the sample of Biodiesel with glycerol at bottom and Fig.1 b) shows the sample with separated glycerol. |
 |
III EXPERIMENTAL SETUP |
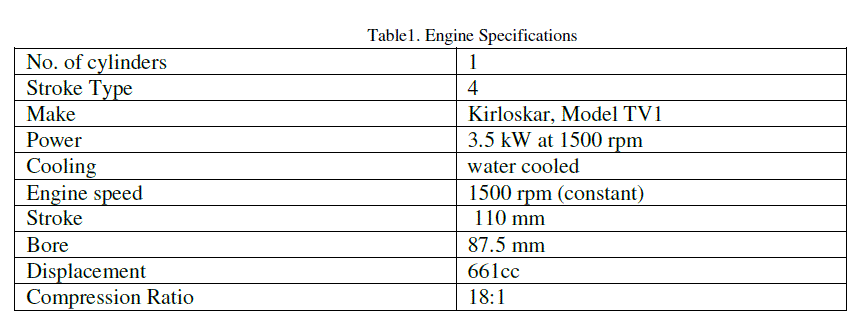
| Both the fuels diesel and 100% Biodiesel (B100) were tested onDiesel engine test rig [6].Table.1 shows the specifications of the engine. |
 |
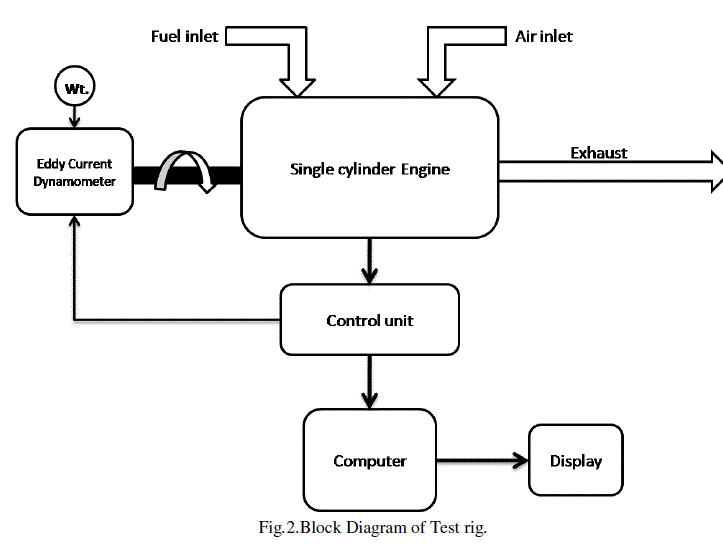
| The performance of engine was first tested on commonly available diesel with values of density and GCV as 0.830 gm/cc and 42,000 KJ/Kg respectively. The compression ratio of the engine was set at 18:1 throughout the testing. The engine was turned on and allowed to run at idling condition with no load up to 15 minutes and then it was tested by varying the load gradually from 0 kg to 16 kg in 9 steps by an eddy current dynamometer connected to the engine. After testing, the leftover diesel was drained off completely from the tank, and was filled with B100 bio-diesel (100% bio-diesel). The engine was started and allowed to run at no load for about 30 minutes, so as to make sure all the diesel in the pipes, hoses and fuel filter is completely replaced by bio-diesel. The engine was again tested for 9 different load values from 0 kg to 16 kg and the values are recorded and saved.Fig 2. shows the block diagram of the experimental setup. |
 |
IV. RESULTS AND COMPARISON |
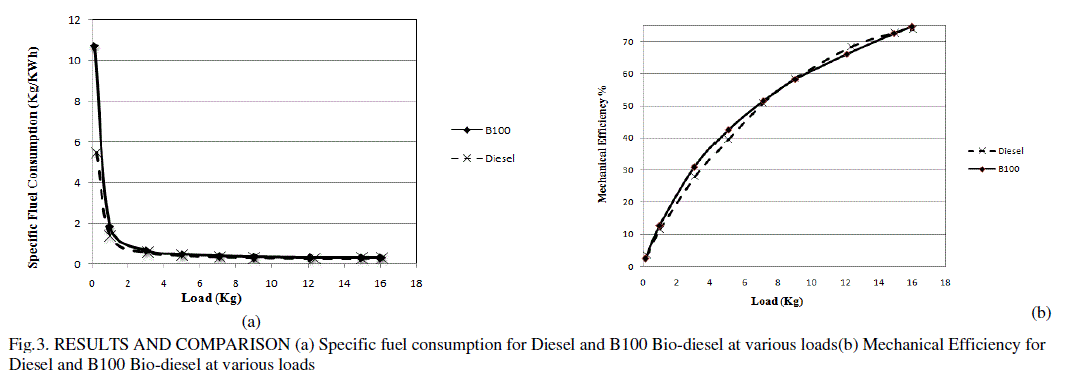
| The graph plotted for specific fuel consumption for diesel and B100 biodiesel is shown in the Fig. 3(a). At lower loads there was a huge variation in SFC value and as the load increased, the SFC for both the fuels came closer. The highest difference was found at the initial load condition with difference of 0.47 Kg/KWh (1.35 Kg/KWh |
| for Diesel and 1.82 Kg/KWh for Bio-diesel). The lowest difference of 0.01 Kg/KWh was found at the highest load (0.29 Kg/KWh for Diesel and 0.3 Kg/KWh for Bio-diesel). |
| The mechanical efficiency of both the fuels was found to be very close especially at higher loads shown in Fig.3 (b) The maximum mechanical efficiency was found to be 74.5% and 73.8% at 16 kg load for B100 and diesel respectively. |
 |
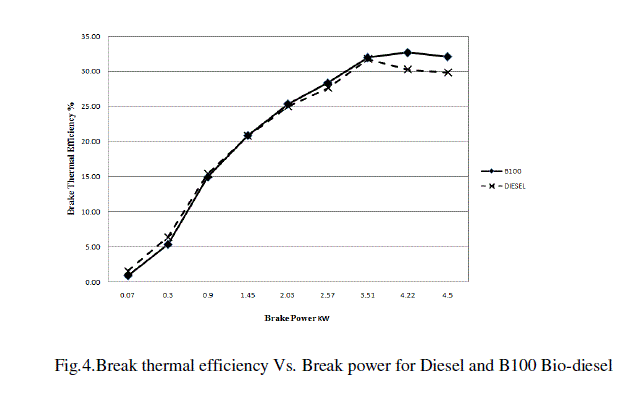
| The BTHE of both the fuels were very close and sometimes overlapping up to a certain load point Fig.4. At higher break power ranging from 3.5kW to 4.5kW, considerable difference was seen. However for Diesel it started decreasing from approximately from 3.5 kW.The maximum BTHE was found to be 32.1% for B100 bio-diesel and 29.8% for diesel at 16 kg load . |
 |
V. CONCLUSION |
| According to the graphs and comparison between diesel and B100 Biodiesel for various parameters, the performance of both the fuels is similar with very little or negligible difference. According to the results obtained, the biodiesel yields higher performance than diesel at higher load conditions. There is a very little difference in power at normal loads for both the fuels. This difference cannot be observed while driving a car with diesel and B100 biodiesel. |
References |
|