ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
| A. S. Fouda*, K. shalabi, N. H. Mohamed Department of Chemistry, Faculty of Science, El-Mansoura University, El-Mansoura-35516, Egypt |
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
The inhibition effect of some Chalcone derivatives [3-(4-hydroxyphenyl)-1-phenylprop-2-en-1-one as an inhibitor (1) and 3-(4-hydroxyphenyl)-1-(4-nitrophenyl)prop-2-en-1-one as an inhibitor (2)] on the corrosion of aluminum in 0.5 M HCl solution was studied using potentiodynamic polarization, electrochemical impedance spectroscopy (EIS), electrochemical frequency modulation (EFM) and weight loss measurements. The results drawn from the different techniques are comparable and exhibit small discrepancy. Results for weight loss indicated that inhibitor efficiency (% IE) increased with increasing inhibitor concentration, reaching a maximum inhibiting power of 85.9 , 95.7 % at 21 x 10-6 M for inhibitors 1 and 2, respectively and decreased with increasing the temperature. Polarization studies clearly revealed that the presence of inhibitors changes the mechanism of hydrogen evolution and the metal dissolution i.e. they act as mixed type inhibitors. Some activation and adsorption thermodynamic parameters were calculated and discussed. The surface coverage of the inhibitors obeyed Langmuir adsorption isotherm.
Keywords |
| Chalcone derivatives, Aluminum, HCl, Potentiodynamic polarization, EIS, EFM, Langmuir isotherm |
INTRODUCTION |
| Aluminum is one of the metals which used in different human activities and many of important applications, where it is the second most abundant metal after iron, it has a low atomic mass and negative value of standard electron potential, aluminum potentially attracts as an anodic material for power sources with high energy density. It is used in construction, packing and transportation because of its strength and electrical conductivity. Aluminum is used in electronics due to it is super purity [1]. Although Al has an adhesive protective passivating oxide film, but this film has an amphoteric susceptibility, and consequently the metal dissolves readily in acidic and basic solutions concentrated above and below pH 4-9 [2, 3]. In our efforts to mitigate electrochemical corrosion of aluminum, the main strategy is to isolate the metal from corrosive agents and this can be achieved by using corrosion inhibitors which prevent the adsorption of the aggressive anions or by the formation of a more resistance oxide film on the metal surface. Generally, it has been assumed that the first stage in the action mechanism of the inhibitors in aggressive acid media as hydrochloric acid is the adsorption of the inhibitors on the metal surface where HCl solutions are used for pickling of aluminum for its chemical or electrochemical etching [4]. Various mechanisms have been proposed to explain the breakdown of the passive oxide film when chloride reach the metal film interface. Recently, has shown that chloride does not enter the oxide film but it is chemisorbed on the oxide surface [5] and act as a reaction partner, aiding dissolution via the formation of oxide-chloride complexes. Among several methods used in combating corrosion problems, the use of chemical inhibitors remains the most cost effective and practical method [6, 7]. From this point of view, a great number of investigators have conducted research work to find effective inhibitors for aluminum corrosion in harsh environments [8-10]. Some reported the effect of inorganic oxidants [8], while others studied the use of organic compounds [11-14]. It is well known that azole derivatives like benzotriazole, mercaptoben, benzimidazole and imidazole can be employed as inhibitors against corrosion for many metals and alloys. Azole compounds have been used to protect the corrosion of aluminum [15] in corrosive environments. In general, organic compounds with oxygen, sulfur, and/or nitrogen as polar groups and conjugated double bonds in their structures have been reported to be good corrosion inhibitors for many metals and alloys in corrosive media [16–32]. The inhibiting action of these organic compounds is usually attributed to their interactions with the metallic surfaces via their adsorption. Polar functional groups are regarded as the reaction center that stabilitizes the adsorption process [6]. However, the adsorption of an inhibitor on a metal surface depends on a few factors, such as the nature and surface charge of the metal, the adsorption mode, the inhibitor’s chemical structure, and the type of the electrolyte solution [33]. In this work, we investigate the effect of some chalcone derivatives on the corrosion behavior of aluminum in 0.5 M HCl solution using chemical, electrochemical techniques and quantum chemical calculations. |
II. EXPERIMENTAL |
| 2.1. Materials and reagents Aluminum sheets with purity more than 98.8 % were used in this study. The aggressive solution, 0.5 M HCl was prepared by dilution with bidistilled water. The chalcone derivatives were synthesized according to the procedures described in previous paper [34] and are presented in Table 1. 10-6 M stock solutions from the investigated compounds were prepared by dissolving the appropriate weights of the used chemically pure solid compounds in absolute ethanol. |
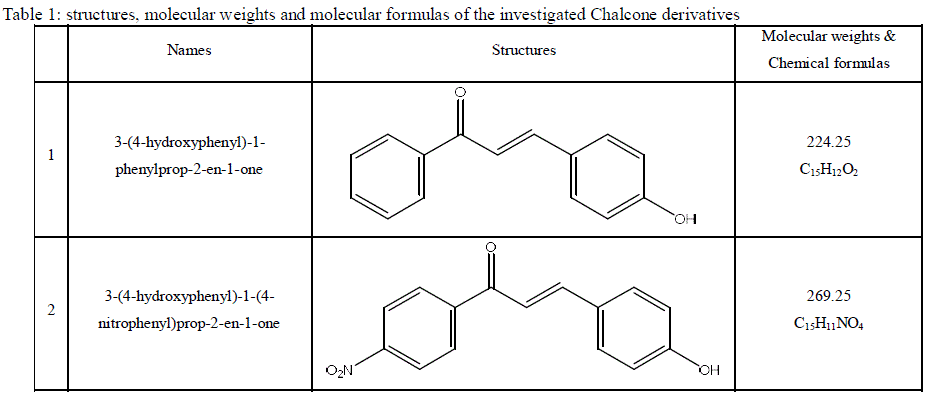 |
| Table 1: structures, molecular weights and molecular formulas of the investigated Chalcone derivatives |
| 2.2. Weight loss measurements The Seven parallel aluminum sheets of (2.4 x 2.5 x 0.1 cm) were abraded with emery paper (grade uo to1200 grit size) and then washed with bidistilled water and acetone. After accurate weighing, the specimens were immersed in a 250 ml beaker, which contained 100 ml of HCl with and without addition of different concentrations of the investigated compounds. All the aggressive acid solutions were open to air and the measurement was conducted at room temperature (25 ± 1 °C). After 5 h, the specimens were taken out, washed, dried, and weighed again accurately. The average weight loss of seven parallel aluminum sheets could be obtained. The inhibition efficiency (% IE) and the degree of surface coverage, θ, of investigated compounds for the corrosion of aluminum in HCl were calculated as follows [35]: % IE = θ x 100 = [(Wo – W)/ Wo] x 100 (1) where Wo and W are the values of the average weight losses in the absence and presence of the inhibitor, respectively. 2.3. Electrochemical measurements The experiments were carried out potentiodynamically in three electrode cell. Platinum foil was used as counter electrode and a saturated calomel electrode (SCE) coupled to a fine Luggin capillary as the reference electrode. The working electrode was in the form of a square cut from aluminum sheet under investigation and was embedded in a Teflon rod with an exposed area of 1 cm2. This electrode was immersed in 100 ml of a test solution for 30 min until a steady state open-circuit potential (Eocp) was attained. The potentiodynamic curves were recorded by changing the electrode potential from -0.5 to 1.5 V versus SCE with scan rate of 1 mV/s. All experiments were carried out in freshly prepared solution at room temperature (25oC) using a thermostat. % IE and the degree of surface coverage (θ) were calculated from Eq. (2): |
| %IE = ï± x 100 = [ 1 – (icorr(inh) / icorr) ] x 100 (2) where (icorr, and icorr(inh) ) are the uninhibited and inhibited corrosion current density values, respectively, determined by extrapolation of Tafel line. The electrochemical impedance spectroscopy (EIS) spectra were recorded at open circuit potential (OCP) after immersion the electrode for 30 min in the test solution. The ac signal was 5 mV peak to peak and the frequency range studied was between 100 kHz and 0.1 Hz. The main parameters deduced from the analysis of Nyquist diagram are the polarization resistance Rp and the capacitance of double layer ( Cdl) which is calculated from Eq.(3): Cdl =1/ (2 π fmax RP ) (3) where (fmax) is the angular frequency at which the imaginary component of the impedance reaches its maximum values. The inhibition efficiency (%IE) and the surface coverage (θ) of the used inhibitors obtained from the impedance measurements were calculated by applying the following relations: % IE = θ x 100 = [1- (Rp ° /Rp)]×100 (4) Where Rp o and Rp are the polarization resistance in the absence and presence of inhibitor, respectively. Electrochemical frequency modulation (EFM) is a non-destructive corrosion measurement technique that can directly give values of the corrosion current without a prior knowledge of Tafel constants. Like EIS, it is a small signal ac technique. Unlike EIS, however, two sine waves (at different frequencies) are applied. The intermodulation spectra contain current responses assigned for harmonically and intermodulation current peaks. The larger peaks were used to calculate the corrosion current density (icorr), the Tafel slopes (βc and βa) and the causality factors CF-2& CF-3 [36,37] . The electrode potential was allowed to stabilize for 30 min before starting the measurements. All the experiments were conducted at 25± 1°. All electrochemical measurements were carried out using Potentiostat /Galvanostat/ Zra analyzer (Gamry PCI300/4). A personal computer with DC105 software for potentiodynamic, EIS300 software for EIS and EFM140 software for EMF and Echem Analyst 5.21 was used for data fitting. 2.4. Surface morphology For morphological study, surface features (2.0 cm x 2.0 cm x 0.15 cm) of aluminum were examined before and after exposure to 0.5 M HCl solutions for 24 hour with and without inhibitor. JEOL JSM-5500 scanning electron microscope was used for this investigation. |
III. RESULTS AND DISCUSSION |
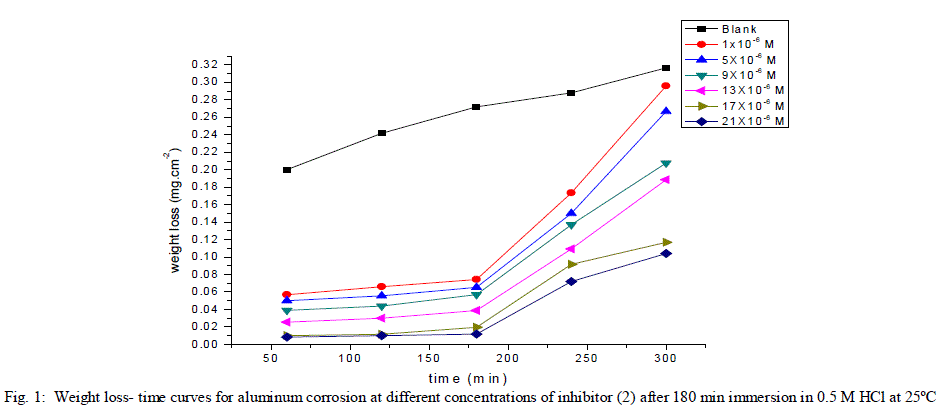
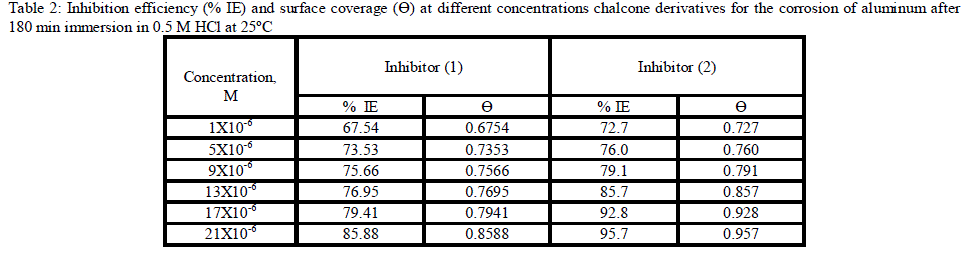
| 3.1. Weight loss measurements Weight The corrosion rate of aluminum as a function of time and temperature were investigated. Fig (1) represents the weight loss-time curves in the absence and presence of different concentrations of inhibitor (2) at 25°C (the more effective inhibitor). Similar curves were obtained for the other inhibitor (not shown). It is obvious that the uniformity and non-linearity of the plot in the absence of inhibitor suggest that the aluminum corrosion in HCl is a heterogeneous process involving several steps. Similar observations have been reported for aluminum corrosion in other media [38]. The corrosion of aluminum in HCl in the presence of inhibitor shifted to more uniform and more linear plots, and is much lower than in case of absence of inhibitor. The inhibition efficiency (% IE) and the surface coverage (θ) that represents the weight of the metal surface covered by inhibitor molecules were shown in Tables (2). The %IE increases with increasing the inhibitor concentration indicated that more inhibitor molecules are adsorbed on the metal surface thus providing wider surface coverage. The optimum concentration required to achieve an efficiency of 95.75% and 85.88% was found to be 21x10-6 M for inhibitors 1 &2, respectively. |
 |
 |
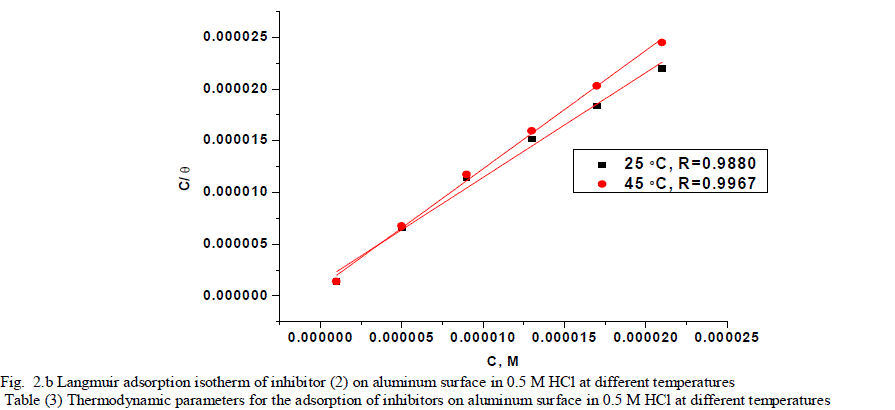
| 3.2. Adsorption isotherms Corrosion inhibitors are found to protect aluminum corrosion in acid solutions by adsorbing themselves on aluminum surface where the adsorption of the organic molecules occurs as the interaction energy between molecule and metal surface is higher than that between the water molecules and the surface [39], and it is regarded as substitution adsorption process between the organic molecules in the aqueous phase (orgaq) and the water molecules adsorbed on the aluminum surface (H2O)ads [40]. |
| xH2Oads + Orgsol ↔ Orgads + xH2O (5) |
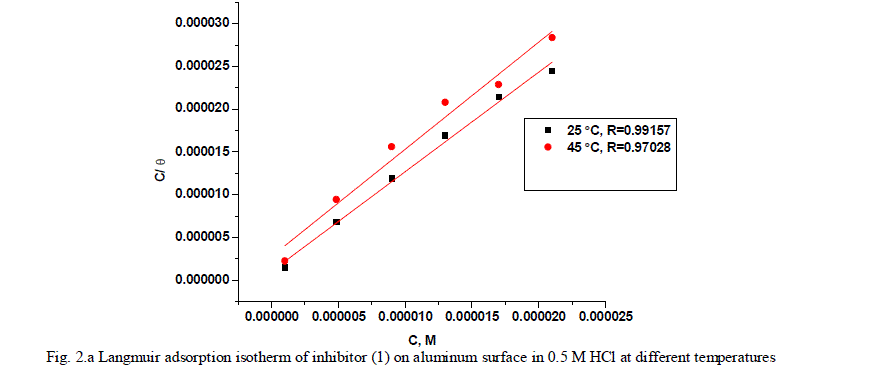
| where x is the size ratio, that is, the number of water molecules replaced by one organic molecule. Inhibitors molecules are either physically or chemically adsorbed on a corroding metal surface, where the physisorbed molecules retard metal dissolution by inhibiting the cathodic reaction whereas chemisorbed molecules inhibit the anodic reaction by reducing the inherent reactivity of the metal at the adsorption sites [41]. It was found that the best suitable adsorption isotherm for the studied inhibitors on the aluminum surface is the Langmuir equation [42] which was defined as follows: |
| C/ÃÂ = 1/Kads + C (6) |
| where Kads is equilibrium constant of adsorption process, and C is concentration of inhibitor. The plot of C/ÃÂ versus C for the inhibitors are shown (Figs. 2a,2b) to be linear with slope near unity, and correlation coefficients near to unity in order to obtain the isotherm. The thermodynamic adsorption parameters were calculated. The well-known thermodynamic adsorption parameters are the standard free energy of adsorption (ΔG°ads), the heat of adsorption (ΔH°ads) and the entropy of adsorption (ΔS°ads). These quantities can be calculated by various mathematical methods depending on the values of Kads from adsorption isotherms at different temperatures [43]. The ΔG°ads can be calculated from Eq. (7): |
| Kads = (1/55.5) exp (-ΔG°ads / RT) (7) |
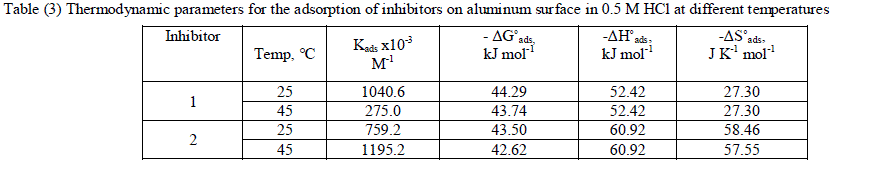
| where 55.5 is the concentration of water in mol l-1, R is the universal gas constant, and T is the absolute temperature. (ΔH°ads), (ΔS°ads) can be calculated from Eqs (8 &9): Log Kads= (ΔH°ads / 2.303RT) + constant (8) ΔG°ads = ΔH°ads - TΔS°ads (9) Table (3) shows all the estimated thermodynamic adsorption parameters for the chalcone derivatives on aluminum surface, and concluded that: Large values of Kads mean good inhibition efficiency of the inhibitors and strong electrical interaction between the adsorbate and the adsorbent. The sign of ΔG°ads was negative which reflects that the adsorption of these inhibitors is spontaneous process. It is well known that values of ΔGÃÂads of the order of 40 kJ mol-1 or higher involve charge sharing or transfer from the inhibitor molecules to metal surface to form coordinate type of bond (chemisorption); those of order of 20 kJ mol-1 or lower means that the electrostatic interaction between metal surface and charged organic molecules in the bulk of the solution indicate a physisorption [44, 45]. The calculated ΔGÃÂads values are above 40 kJ mol-1 which indicate that the adsorption mechanism of the investigated compounds on aluminum in 0.5 M HCl solution is a chemisorption and shifted to physisorption by increasing temperature, so it is a comprehensive adsorption (physical and chemical). ΔG°ads values increase (become less negative) with an increase in temperature which indicate that the adsorption process is an exothermic process. The negative sign of ΔH°ads indicates that the adsorption process of both inhibitors molecules is an exothermic process. Generally, an exothermic adsorption process suggests either physisorption or chemisorption while endothermic process is attributed to chemisorption [46]. Generally, enthalpy values up to 41.9 kJ.mol-1 are related to physisorption while those around 100 kJ.mol-1 or higher are attributed to chemisorption. The unshared electron pairs in investigated molecules may interact with p-orbital of aluminum to provide chemisorbed film.The values of ΔS°ads are large and negative that is accompanied with exothermic adsorption process. |
 |
 |
 |
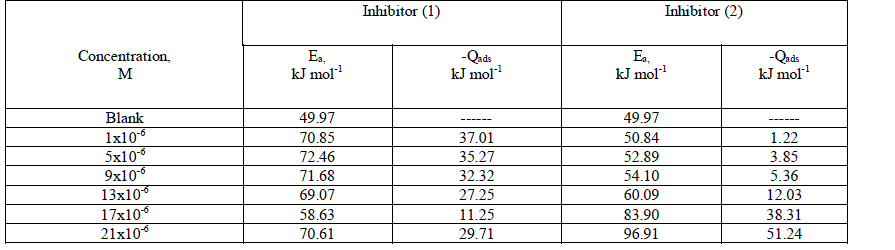
| 3.3. Effect of temperature The importance of temperature variation in corrosion study involving the use of inhibitors is to determine the mode of inhibitor adsorption on the metal surface. Recently, the use of two temperatures to establish the mode of inhibitor adsorption on a metal surface has been reported and has been found to be adequate. Thus, the influence of temperature on the corrosion behavior of aluminum in 0.5 M HCl in the absence and presence of the investigated compounds by hydrogen evolution method at 25 and 45 °C. Therefore, in examining the effect of temperature on the corrosion process, the apparent activation energies (Ea) were calculated from the Arrhenius equation [47]. Log (k2 corr / k1 corr) = (Ea /2.303R) x (1/T1 -1/T2) (10) where k1corr and k2corr are the corrosion rates at temperature T1 and T2, respectively. An estimate of heat of adsorption was obtained from the trend of surface coverage with temperature as follows [48]: Qads = 2.303R [log (ÃÂ2/1- ÃÂ2) – log (ÃÂ1/1- ÃÂ1)] x (T1 T2 / T2 - T1) (11) where ÃÂ1 and ÃÂ2 are the degrees of surface coverage at temperatures T1 and T2, The calculated values for both parameters are given in Table 4. Increased activation energy (Ea) in inhibited solutions compared to the blank suggests that the inhibitor is physically adsorbed on the aluminum metal surface [49]. It is seen from Table 4 that Ea values rose with increasing the inhibitor concentration, suggesting strong adsorption of the investigated inhibitors on the aluminum metal surface. It has been suggested that adsorption of an organic inhibitor can affect the corrosion rate by either decreasing the available reaction area (geometric blocking effect) or by modifying the activation energy of the anodic or cathodic reactions occurring in the inhibitor-free surface in the course of the inhibited corrosion process [50] The negative sign Qads values indicate that the adsorption process and the degree of surface coverage decreased with increasing in temperature, supporting the physisorption mechanism [51]. Table 4: The activation energy (Ea) and the heat of adsorption (Qads) for aluminum dissolution in 0.5 M HCl in the absence and presence of inhibitors at 25 and 45°C |
 |
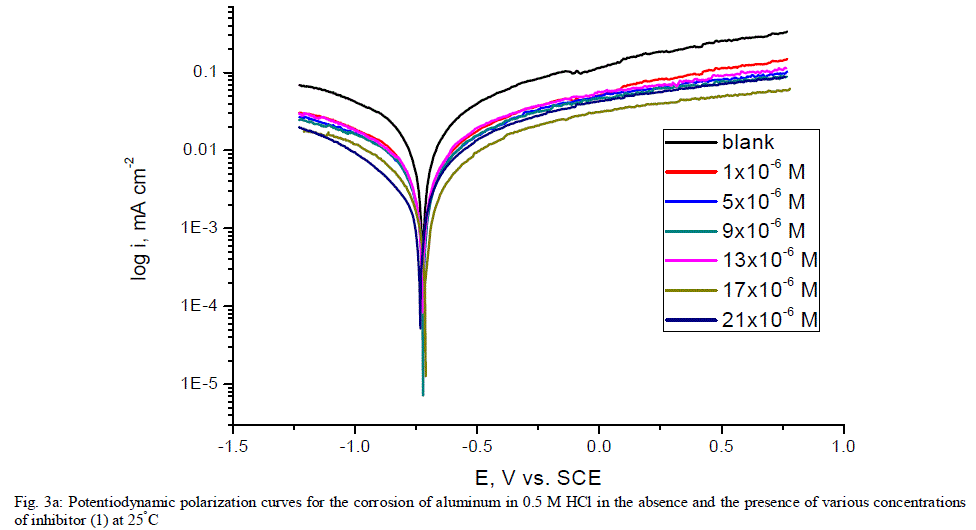
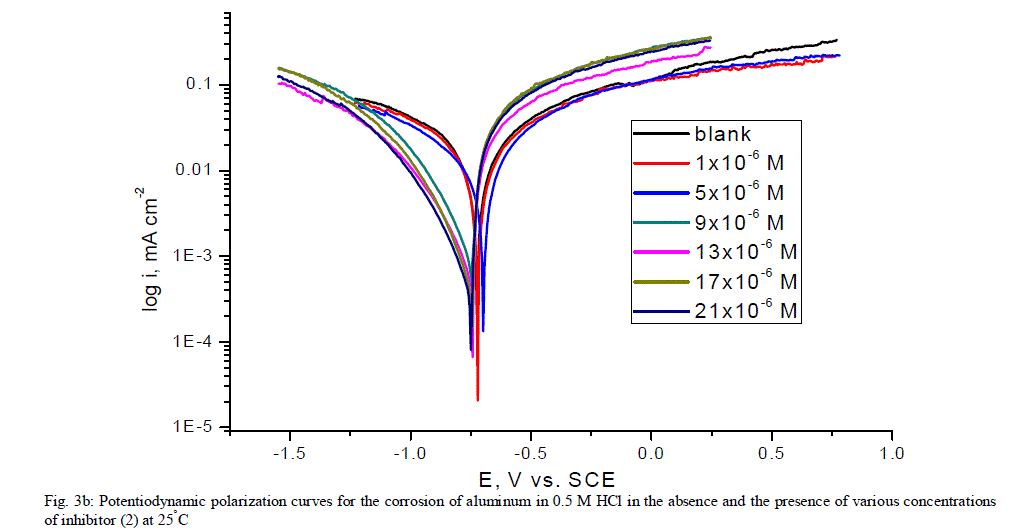
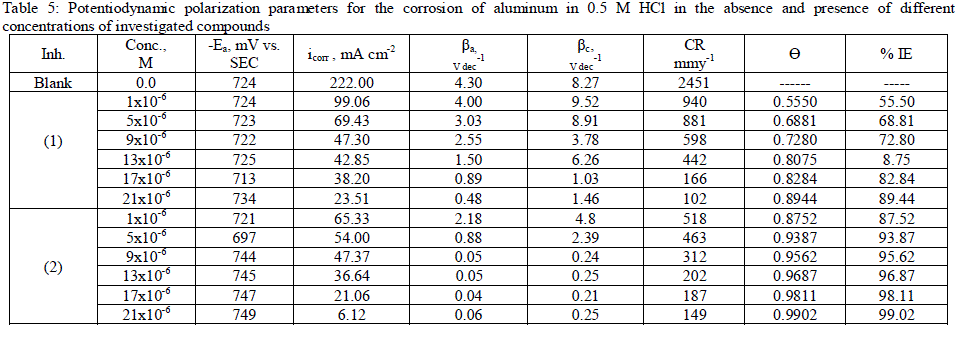
| 3.5. Potentiodynamic polarization measurements The potentiodynamic polarization curves of aluminum in 0.5 M HCl solutions containing different concentrations of investigated compounds at 25°C are shown in Figs. (3.a, 3.b) for inhibitors 1, 2 respectively. The corrosion kinetic parameters such as corrosion current density (icorr), corrosion potential (Ecorr.), the anodic Tafel slopes (βa) and cathodic Tafel slope (βc), degree of surface coverage (ÃÂ) and the inhibition efficiency (%IE) of both inhibitors are shown in Table 5 for the aluminum in 0.5 M HCl solution in the absence and presence of different concentrations of the both inhibitors are listed in Table 5. The results of this Table indicated that the corrosion current density (icorr) decreases in the presence of inhibitors compared to the blank solution and also with increasing the inhibitors concentrations which suggest that the presence of these compounds retard the dissolution of aluminum in 0.5 M HCl solution. Presence of these inhibitors cause decrease in the corrosion rate, so shift both the anodic and cathodic curves to lower values of current densities. This implies that both the hydrogen evolution and the anodic dissolution of aluminum metal are inhibited. This due to adsorption of the inhibitors over the corroding surface [52, 53]. The slopes of the anodic and cathodic Tafel lines (βa, βc) were slightly changed on increasing the concentration of the investigated compounds. This means that there is no change of the mechanism of the inhibition in presence and absence of inhibitors and that inhibitors affects both anodic and cathodic reactions, so it is mixed type inhibitors. Inhibition efficiency (%IE) was calculated from equation (2). The order of % IE was found to decrease in the following sequence: inhibitor 2 ÃÂ inhibitor 1. |
 |
 |
 |
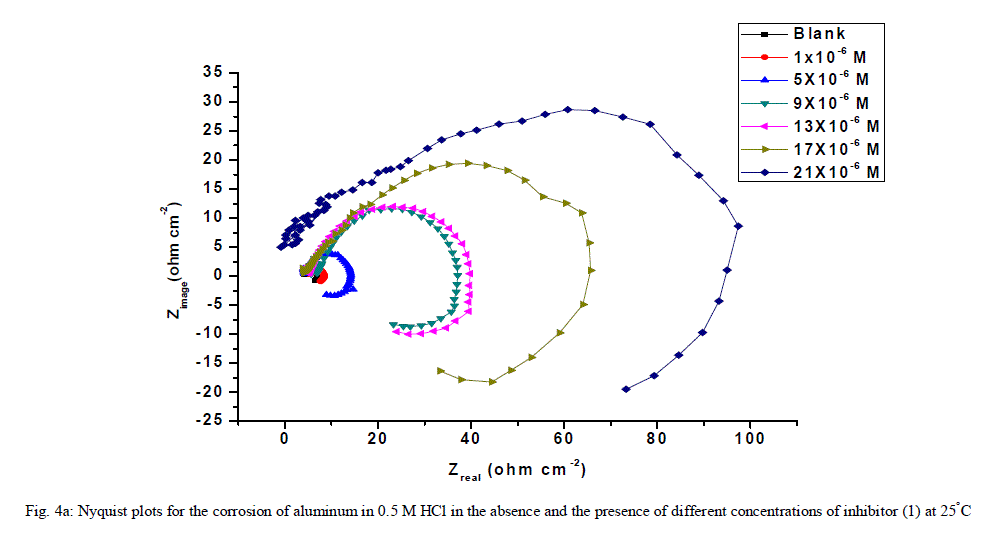
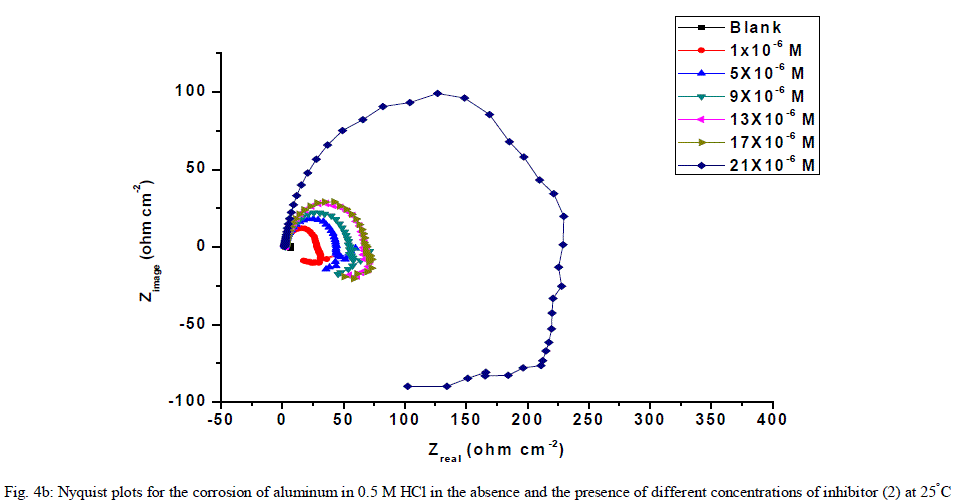
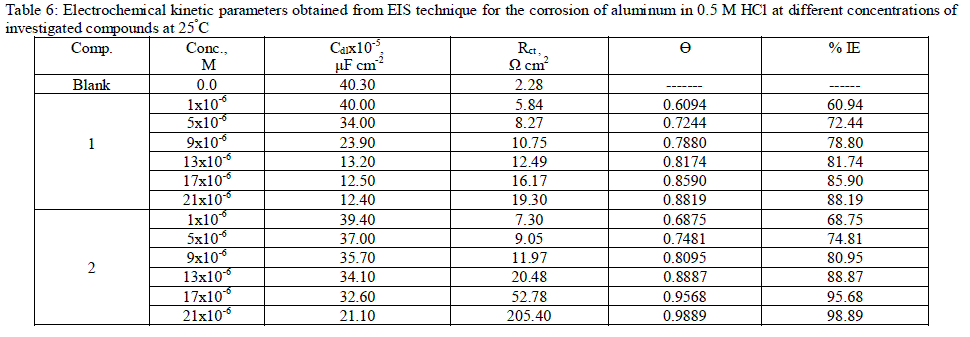
| 3.6. Electrochemical impedance spectroscopy (EIS) measurements The corrosion behavior of aluminum in 0.5 M HCl solution in the absence and presence of different concentrations of the inhibitors was investigated by the EIS method at 25°C after 30 min of immersion. Figs. (4.a, 4.b) show the Nyquist plot for aluminum in 0.5 M HCl solution in the absence and presence of different concentrations of investigated compounds. The fact that impedance diagrams have an approximately semi-circular appearance shows that the corrosion of aluminum in 0.5 M HCl is controlled by a charge transfer resistance process. Small distortion was observed in some diagrams, this distortion has been attributed to frequency dispersion [54] as a result of surface roughness, impurities, dislocations, grain boundaries, adsorption of inhibitors and formation of porous layers and inhomogeneity of the electrode surface. The electrical equivalent circuit model which describes the metal / electrolyte interface of the present corroding system is shown in Fig. 5. The model consists of the solution resistance (Rs), the charge-transfer resistance of the interfacial corrosion reaction (Rct), and the double layer capacitance (Cdl). Table 6 shows the EIS data where the Cdl values decrease and the Rct values increase with the increase of the inhibitor concentrations. This is due to the gradual replacement of water molecules by the adsorption of the inhibitor molecules on the metal surface, and decreasing the extent of dissolution reaction. The high (Rct) values are generally associated with slower corroding system [55, 56]. The decrease in the Cdl can result from the decrease of the local dielectric constant and/or from the increase of thickness of the electrical double layer [57] suggested that the inhibitor molecules function by adsorption at the metal/solution interface. The % IE obtained from EIS measurements are close to those deduced from polarization method. The order of inhibition efficiency obtained from EIS measurements is as follows: inhibitor 2 ÃÂ inhibitor 1 |
 |
 |
 |
 |
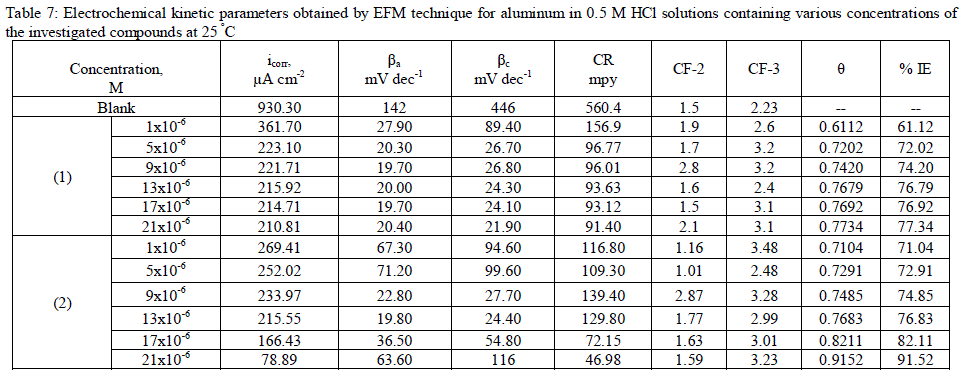
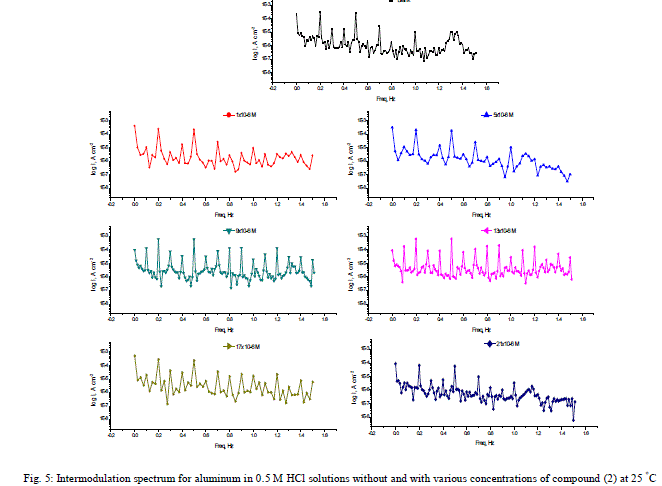
| 3.7. Electrochemical frequency modulation (EFM) measurements The EFM is a nondestructive corrosion measurement technique that can directly give values of the corrosion current without prior knowledge of Tafel constants. It is generally accepted that in most cases, the corrosion rates determined with the EFM technique, are much higher than the values determined with other techniques exhibiting low corrosion rates (58). The modulation frequencies that are used in the EFM technique are in the capacitive region of the impedance spectra. However, results of the EFM technique showed good agreement of corrosion rates obtained with the Tafel extrapolation method. Figs. 5 are example of aluminum immersed in 0.5 M HCl solutions and in presence of 21 x10-6 M inhibitor (2) as an example at 25°C. Each spectrum is a current response as a function of frequency. Table 5 shows the calculated corrosion kinetic parameters at different concentrations of the investigated compounds in 0.5 M HCl at 25°C (icorr, βa, βc, CF-2, CF-3 and % IE). From Table 4, the corrosion current densities decrease by increasing the concentration of investigated compounds and the efficiency of inhibition increases by increasing investigated compounds concentrations. The causality factors in Table 7 are very close to theoretical values, which according to EFM theory [59] should guarantee the validity of Tafel slopes and corrosion current densities. Values of causality factors in Table 7 indicate that the measured data are of good quality. The standard values for CF-2 and CF-3 are 2.0 and 3.0, respectively. The deviation of causality factors from their ideal values might be due to the perturbation amplitude which was too small or that the resolution of the frequency spectrum is not high enough. Another possible explanation is that the inhibitor is not performing very well. The obtained results showed good agreement of corrosion kinetic parameters obtained with the EFM, Tafel extrapolation and EIS methods. |
 |
 |
| 3.7.2. SEM Characterization Figure 6 shows the scanning electron microphotographs of aluminum in HCl in the presence of 21 x10-6 M of investigated compounds. As cn be seen from this Figure, there are distinct differences between the the four aluminum sheets after the corrosion in acid solution. As for bar aluminum , the surface was seriously damaged as a great deal of deep cavities and drawbach were found. Under the same corrosion circumstance, the surface of aluminum was smooth with a few small notches. The phenomenon implied that the presence of inhibitors films can protect the aluminum from corrosion effictently. On comparing these microphotographs, it appears that maximum smoothing of the surface of test materials has been observed in presence of compound (2) followed by compound (1). This observation is in conformity with the observed inhibition efficiency values as discussed earlier. |
 |
| 3.7 Mechanism of Corrosion Inhibition In general, these inhibitors may be adsorbed on aluminum surface in their neutral or protonated forms (cationic form). Since it is well known that the aluminum surface is negatively charged in acid solution [60], it is easier for the protonated molecules to approach the negatively charged aluminum surface due to the electrostatic attraction. In case of adsorption, this involves the displacement of water molecules from the aluminum surface and sharing electrons between the hetero-atoms and aluminum. Also, the inhibitor molecules can absorb on aluminum surface on the basis of donor-acceptor interactions between π-electrons of aromatic rings and vacant p-orbitals of surface aluminum atoms. Thus, we can conclude that inhibition of aluminum corrosion in HCl is mainly due to electrostatic interaction. The decrease in inhibition efficiency with rise in temperature (Table 7) supports electrostatic interaction. The order of decreasing inhibition efficiency of the compounds from all techniques used is: inhibitor 2 ÃÂ inhibitor 1. Inhibitor 2 has the highest percentage inhibition efficiency, this being due to its larger molecular size than compound (1) which cover larger area from aluminum surface. Also, NO2 group in compound (2) may reduce in HCl and the secondary product is the compound which adsorbed on aluminum surface. |
IV.CONCLUSIONS |
| The investigated compounds are good inhibitors for the corrosion of aluminum in HCl solutions and they act as mixed type inhibitors. The results obtained from all the electrochemical measurements show that the inhibition properties increase with small differences in their %IE numerical values. Double layer capacitances decrease with respect to blank solution when these compounds were added. This fact may explained by adsorption of these molecules on the aluminum surface. The adsorption of the investigated chalcone derivatives on aluminum surface in HCl solution follows Langmuir adsorption isotherm. The negative values of ΔG°ads show the spontaneity of the adsorption process. |
References |
|