ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Binapani Goswami1 and Ranjit Singha2
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
In the present work the synthesis of undoped and Mn- doped ZnO nanoparticles by simple coprecipitation method is reported. The nanoparticles were characterized by transmission electron microscopy (TEM), Xray diffraction (XRD), UV-Vis spectroscopy and Photoluminescence (PL) spectroscopy for their structural and optical properties. X-ray diffraction data revealed the formation of hexagonal wurtzite phase for both undoped and Mn-doped nanoparticles. The mean crystallite size as obtained by Debye-Scherrer formula for undoped and doped ZnO nanoparticles of different concentrations are 26.64 nm, 21.66 nm, 23.60 nm and 22.31 nm respectively. The broadening in the XRD patterns clearly indicates the presence of very small nanocrystals. A linear decrease in the band gap is observed with increase of doping percentage. The room temperature PL spectra reveal that all the particles have a high purity with perfect crystallinity; however some crystal defects (vacancy, interstitial etc) are also present.
Keywords |
| ZnO, co-precipitation method, TEM, XRD, UV-Vis spectroscopy, band gap, PL etc. |
INTRODUCTION |
| In recent years ZnO nanostructures have been extensively studied due to their versatile properties and their potential for the development of novel nanodevices. The excellent properties possessed by ZnO such as a wide direct band gap (3.37 eV at 300K), a large exciton binding energy of 60 MeV and a large melting point of 1975 0C make it suitable for application in photodetectors [1,2], light emitting diodes [3,4], laser light source [5] and nanosensors [6,7]. The modification of the properties of ZnO by incorporation of dopant has become an important research topic in recent days. Doping of various elements in suitable amounts is found to enhance optical, electrical and magnetic properties of the particles by altering its electronic structure and band gap [8]. |
| In this paper we demonstrate successful synthesis of undoped and Mn doped ZnO nanoparticles by simple coprecipitation technique. The morphology and crystallinity of the as prepared particles were carefully investigated by employing various characterization techniques XRD, TEM etc. We discussed the detailed optical properties of both doped and undoped particles based on UV-Vis analysis and room temperature Photoluminescence (PL). |
MATERIALS AND METHODS |
Synthesis |
| To prepare undoped ZnO nanoparticles, following steps were undertaken: 0.05M zinc acetate dihydrate [Zn(CH3COO)2,2H2O] and 0.4M Sodium hydroxide in 100ml distilled water were prepared and mixed together by constant stirring at 70°C for 30 minutes with the help of a magnetic stirrer. During this 2wt% PolyVinylPyrolidone (PVP) i.e. 2gm PVP in 100ml distilled water at 70°C was added drop wise to the above mixture. The precipitates which get formed are separated from the solution by filtration, washed several times with deionized water and absolute ethanol and then dried in atmosphere for several days to obtain ZnO nanoparticles in powder form. |
| For Mn doped ZnO nanoparticles with Mn content: 2wt%, 4wt% and 6wt%, 0.05M zinc acetate dihydrate and 2wt%, 4wt% and 6wt% Manganese acetate tetra hydrate solution in distilled water were mixed together and stirred at 70°C for 2 hrs. 0.4M Potassium hydroxide solution in deionized water at same temperature was prepared and added drop wise to the above solution. The precipitate which get formed were separated from the solution by filtration, washed several times with distilled water and absolute ethanol and then dried in atmosphere for several days to obtain Mn doped ZnO nanoparticles. |
Characterization |
| The size and morphology of the particles are characterized by transmission electron microscope (TEM) [Model JEOL, JEM-2100] operating with an acceleration potential of 200kV while their crystal structure was determined by X-ray diffraction (XRD) pattern recorded using Brukar D8 Advance powder diffractometer with Cu K∞ radiation working at 40kV. Room temperature photoluminescence spectra were recorded by F-4500 FL spectrophotometer at excitation wavelength 310 nm. The average crystallite sizes were calculated by using by using Debye- Scherrer equation D =k λ/ βcosò, Where D is the diameter of the crystallite, K is the shape factor (the typical value is 0.9), λ is the wavelength of incident beam, β is the broadening of the diffraction line measured in radians at half of its maximum intensity (FWHM), and ò is the Bragg’s angle. |
RESULTS AND DISCUSSIONS |
Structural Analysis |
| Figure 1 shows the XRD patterns of ZnO and ZnO: Mn nanoparticles of different concentrations. The spectrum shows three broad peaks for ZnO and ZnO: Mn at 2ò values 31.70°, 34.39°, 36.22°; 31.740, 34.37°, 36.22°; 31.68°, 34.33°, 36.20° and 31.72°, 34.37°, 36.21° positions respectively. The three diffraction peaks corresponds to the (100), (002) and (101) crystalline plane of hexagonal ZnO. For all the compositions formation of only ZnO wurtzite phase is observed. The mean crystallite size as obtained by Debye-Scherrer formula for undoped and doped ZnO nanoparticles of different compositions are 26.64 nm, 21.66 nm, 23.60 nm and 22.31 nm respectively. The crystallite size calculated from (002) reflections were higher than those calculated from (100) and (101) reflections for all samples. This indicates a small elongation of the crystallites in the direction of the c-axis and attributed to the hexagonal wurtzite structure [9].In the diffraction pattern no additional peaks are observed which might belong to Mn related secondary phases. It may be because Mn atoms are located at substitutional sites for lower doping concentration [10]. The broadening in the XRD pattern clearly indicates the presence of very small nanocrystals. |
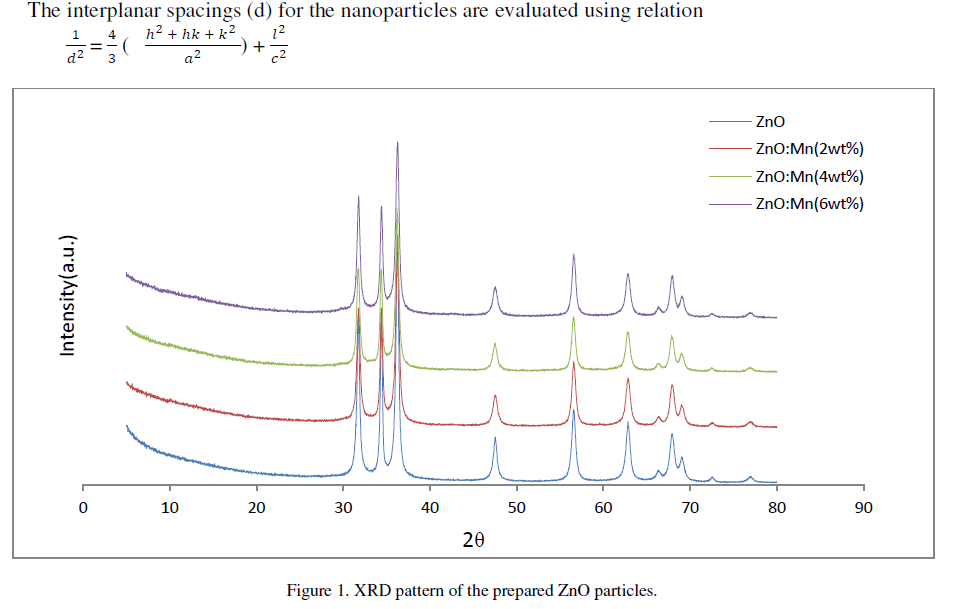 |
| The interplanar spacings of the particles are 2.63 angstrom lattice constants a, b and c is same for undoped and Mn doped ZnO of all concentrations. The results obtained from XRD analysis are summarized in table 2 shown below. |
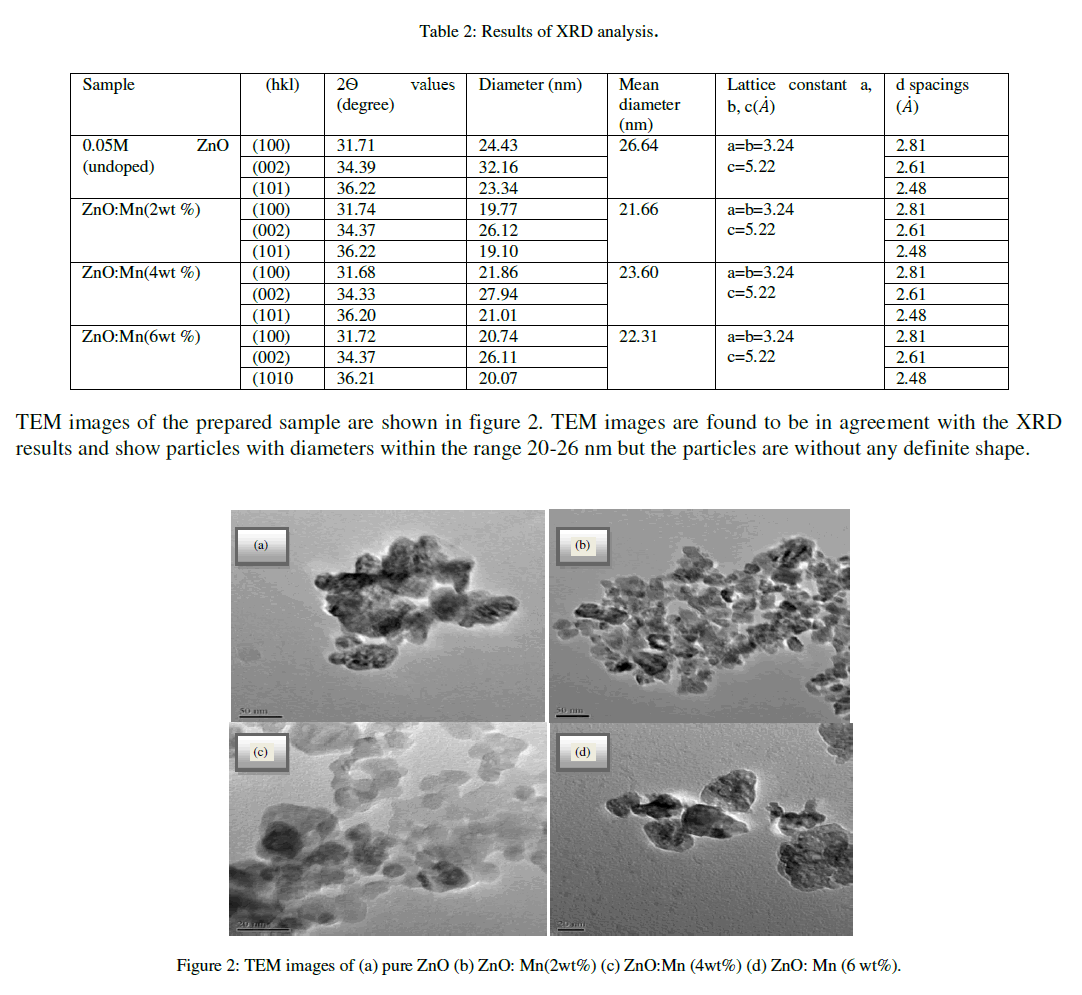 |
| The UV-Vis absorption spectra of ZnO and ZnO:Mn nanoparticles are shown in figure 3. A significant increase in the absorption intensity is seen with the increase of doping concentration. This demonstrates that the Mn doped samples absorbs more visible light and therefore can be used as efficient photo catalyst [11]. Distinct absorption peaks are seen at wavelength around 360nm for undoped particles and 365nm, 370nm and 374nm respectively for Mn doped particles. The absorption peak clearly shifts to longer wavelength region with Mn doping. Olguin et.al. [12] reported that the red shift is due to the increase in lattice parameters of the particles. Also,S. Chattopadhyay et.al. [13] related this red shift to the band bending. But the lattice parameters in our case do not show significant variation and it seems that the observed decrease in band gap may not originate from lattice parameter changing. In our work the decrease in band gap may be due to the sp-d exchange interactions and d-d transitions which |
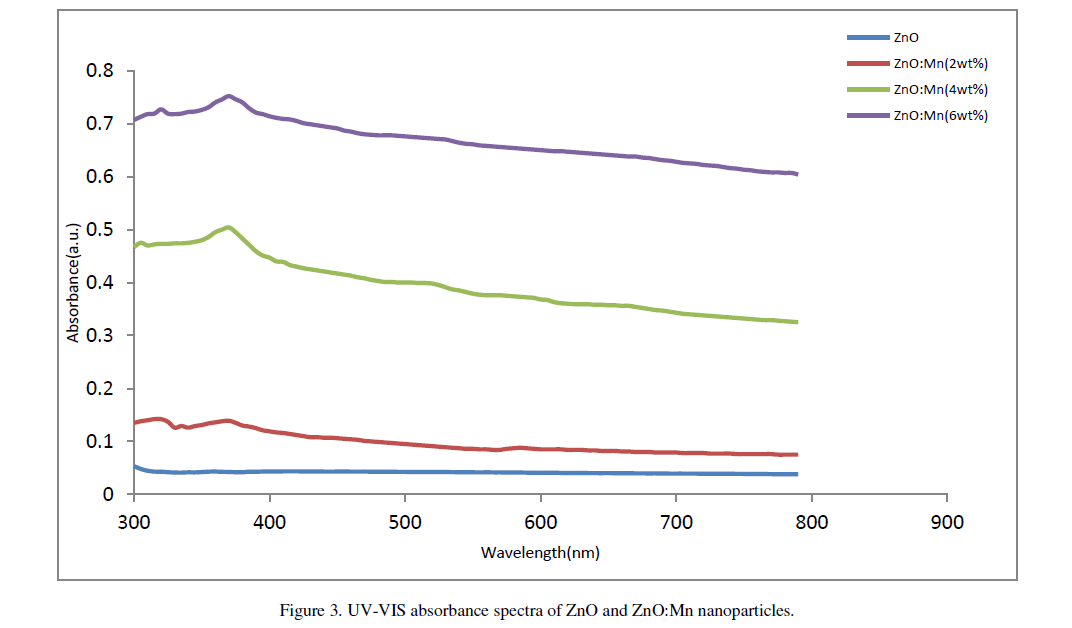 |
| cause the extension in ferromagnetic ordering of the samples, as reported by Kim et.al. [14]. Corresponding band gap of the particles are 3.44eV, 3.39eV, 3.35eV and 3.32eV respectively. |
| The room temperature PL spectra of the prepared samples at excitation wavelength 310 nm are shown in figure 3. It is found that PL spectra of all the samples show four main emission peaks namely UV, violet, blue and green. In ZnO UV emission corresponds to the near band edge emission originating from the recombination of free excitons.[15]. Prominent violet peaks can be assigned as the excitonic recombination between electrons localized at the Zn interstitial and holes in the valence band. The green emission corresponds to the singly ionized oxygen vacancy in ZnO [16]. Blue emission could originate from transitions involving zinc interstitial [17] or possibly due to surface defects in the particles [18]. In our samples the UV peak is at around 393nm, violet peak is at around 456nm, blue peak is at around 492nm and green peak at around 540nm. The PL spectrum also exhibits one additional weak peak at around 430nm. This PL signal is attributed to band-edge free excitons [19]. |
| The increase of percentage of Mn doping has a direct effect on the intensity of visible emissions. A systematic increase in visible emissions is observed with an increase of doping concentration up to 4wt% of Mn doping. But it decreases slightly for 6wt% Mn doping. Thus in our case maximum emission is observed in case of 4wt% Mn doping. |
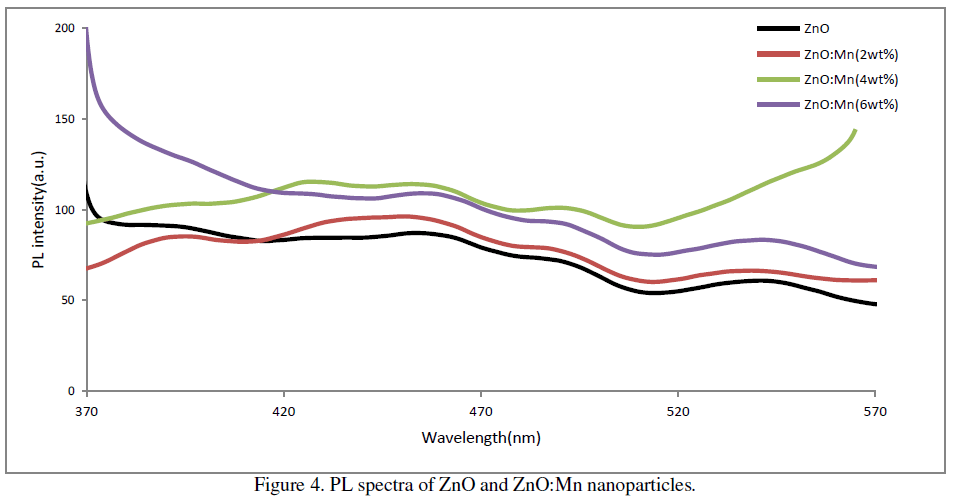 |
CONCLUSIONS |
| We have successfully fabricated undoped and Mn doped ZnO nanoparticles by co-precipitation method. The fabricated nanoparticles are found to have hexagonal wurtzite structure with particle sizes in the range 21-26 nm and without any definite shape. Bang gap of the particles decrease with the increase in doping percentage. Three different emissions in blue, green and violet regions were visible in the room temperature photoluminescence spectra of the samples excited at 310 nm wavelengths. These visible emissions are attributed to the surface defect, oxygen vacancy and zinc interstitial type of defects. 4wt% Mn doped ZnO particles had the best optical properties in our study. |
ACKNOWLEDGEMENT |
| The authors sincerely acknowledge IASST Gorchuk, Assam, SAIF, NEHU, Shillong and Department of Physics, Tezpur University for providing necessary characterization facility. We also thank Mr. Sudip Das for his valuable help in carrying out the characterizations. |
References |
|