ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Sheeba.E1., S.Palanivel2 and S.Parvathi2
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Different concentrations and combinations of hormones were used in MS medium to observe callus induction and then plant regeneration using leaf as explant. The rate of callus formation varied in different treatments used. The highest amount of callus (80.67%) was produced on MS medium containing 3.0 mg/l NAA from leaf after 20 days. The highest fresh weight of callus was obtained from 2.0 mg/l IAA and 0.5 mg/l BAP. The highest percentage of regeneration (46.28%) was recorded in MS media containing 2.0 mg/l BAP + 0.25 mg/l IAA from leaf after 42 days. The protocol in the study might be useful for the production of disease free and healthy plant materials and also it would be useful for genetic transformation and secondary metabolite production of Physalis minima using biotechnological approach
Keywords |
| Physalis minima, callus, growth regulators, indirect plant regeneration. |
INTRODUCTION |
| The biotechnological approach such as tissue cultures initiated for medicinal plants is a viable method for the production of therapeutic compounds. Plant tissue cultures are at present attracting worldwide attention, because plant cells are able to synthesize specific compounds, especially various secondary metabolites useful as medicines and food additives. Solanaceous plants are able to synthesize a wide range of alkaloids with interesting pharmaceutical activities (Yamada and Tabata, 1997; Alireza et al.2005). Obtaining these alkaloids through in vitro culture techniques remains the focus of considerable research (Robins et al. 1991). Physalis (Ground –Cherry) produces an edible fruit enclosed in bladder like persistent calyx the husk, the name husk tomatoes. The medical uses of Physalis are numerous, a wide variety of species are used for asthma, urinary problems, rheumatism, and tumors (Melissa et al.2005). Physalis minima Linn. is one of the important medicinal plant species belonging to the family Solanaceae and are bitter, appetizing, tonic, diuretic, laxative, and useful in inflammations, enlargement of the spleen and abdominal troubles. The leaves are crushed and applied over snakebite site (Karthikeyan and Janardhanan, 2003).The fruit is considered to be a tonic, diuretic and purgative. The mundas (a tribe) of Chhotaa Nagpur mix the juice of the leaves with water and mustard oil and use it as a remedy against ear ache. The ripen fruits are eaten fresh and generally receive good marks for their taste. An additional benefit is that they are high in vitamin C. The capability to regenerate and propagate plants from cultures cells and tissues is one of the most exciting and useful aspects of in-vitro cell and tissue culture. Increasing demand of those plants, which are specially use for the food and medicine, is one of the cause of their rapid depletion from the natural habitats. |
MATERIALS AND METHODS |
| 1. MS medium was used for callus induction. |
| 2. Physalis minima Linn. was the plant material used in the present study. |
| From the field grown plants, the young leaves were excised out and used for callus induction. The collected explants were washed thoroughly in running tap water for 30 minutes. Then the explants were rinsed with 1% savlon solution containing 6-8 drops of tween 20 for 15 minutes and again washed with double distilled water to remove the traces of detergent solution. Then the explants washed with 70% alcohol for 30 seconds followed by washing with distilled water and the explants were placed inside the laminar air flow chamber. Next, the explants rinsed with 0.05% mercuric chloride solution for 5 seconds and again washed with sterile distilled water for 6-8 times. Then the explants were placed in sterile petriplates for inoculation. The sterilized leaves were used as explant source for in vitro culturing. Murashige and Skoog (1962) medium with various combinations of auxins and cytokinins was used for callus induction. The MS medium consists of macronutrients, micronutrients, iron source and vitamins supplemented with sucrose (3%) as a carbon source and agar (1%) as a solidifying agent.Two cytokinins, 6-Benzyl amino purine and kinetin, and three auxins, Indole-3- acetic acid, 2,4 –Diphenoxy acetic acid and Naphthalene-3-acetic acid in different concentrations were also supplemented in to the MS medium for callus induction. The cytokinin concentrations were constant (0.5 mg/l). Auxin concentrations were ranged from 0.5 mg/l - 3.0 mg/l. Callus cultures were maintained on solid MS medium and sub cultured with frequent intervals and used for further studies. |
RESULTS |
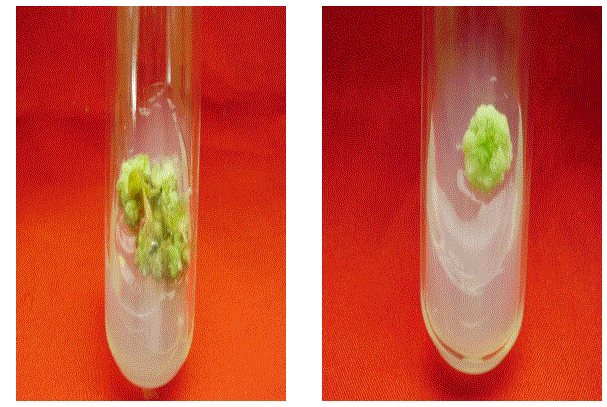
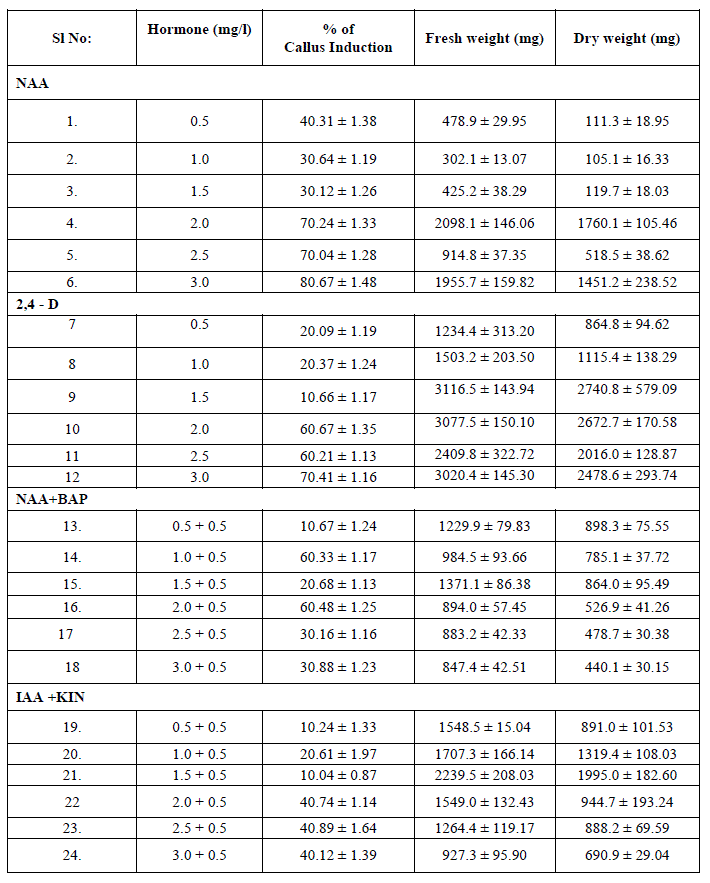
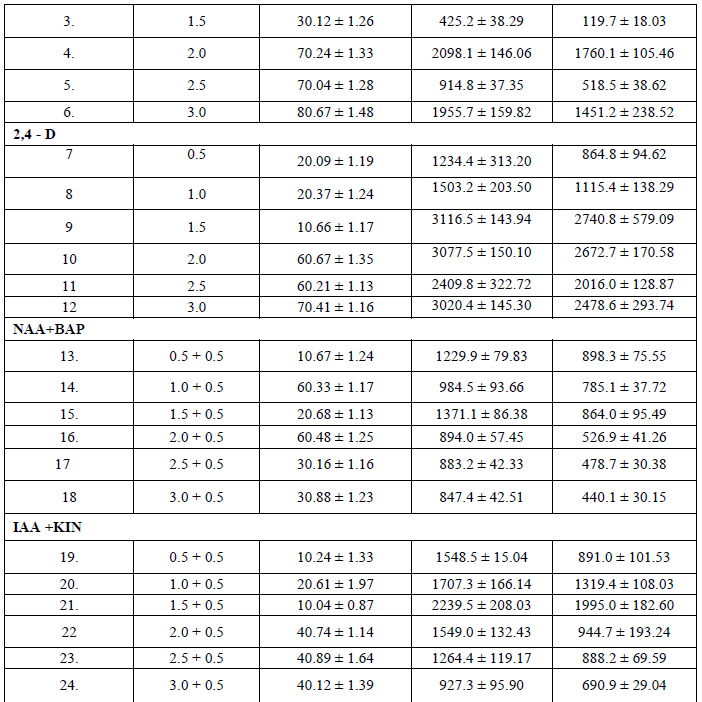
| The ability of callus formation depends on explant source and influenced by type of growth regulator in their concentrations and combinations in the growth medium. Leaf explants were cultured on MS medium with different concentrations of NAA AND 2, 4 –D alone or in combination with BAP and KIN for callus induction. The best result in terms of percentage of callus induction (80.67) obtained on NAA (3.00 mg/l) using leaf as explants after 20 days of inoculation (Table I). Callus obtained from leaf were white and friable (Figure -I). From the different concentrations of hormones 1.50 mg/l IAA and 0.50 mg/l KIN was induced minimum callus production. Out of twenty four hormonal concentrations, 1.5 mg/l 2,4 –D maximum growth in terms of fresh and dry weight of young leaflets callus were 3116.5 mg and 2740.8 mg respectively. |
 |
 |
 |
DISCUSSIONS |
| In general, media containing high auxin and low cytokinin concentrations promote cell proliferation resulting in callus formation (Chawla and Anuja, 2005). In the present research work, an efficient and reproducible protocol has been standardized for callus induction in leaf and nodal explants of Physalis minima. Results obtained from this experiment revealed that the response of explant varies with their hormonal requirement and their concentration for callus induction. In the present study, NAA was effective to produce callus with MS medium and. NAA and BAP, IAA and KIN also showed best results. The result obtained were agreement with the previous reports by other investigators, Solanum tuberosum (Mutasim et al. 2010, Shamima et al. 2003), Solanum surattense (Swarnkar et al. 1986). MS medium with growth regulators was suitable for callus production in most of the Solanaceae family members (Mutasim et al. 2010; Nistor et al. 2009; Swarnkar et al. 1986). In contrast to present study, 2, 4 –D and BAP combination was effective for callus induction from Solanum tuberosum (Mutasim et al. 2010; Nistor et al. 2009). |
CONCLUSION |
| Callus can use for secondary metabolite production, genetic transformation studies and indirect micripropagation. Though the plant has immense medicinal value it is gradually declining from the nature due to over exploitation and environmental pollution. Fast forest cleaning activities leading to a depletion of valuable plant resources; the conservation of this valuable genotype is imperative. That is why there is an urgent need of replenishment of the short supply and conservation of this plant resource. |
References |
|