ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
|
Salah Abdulla Hasoon Assistant professor, Dept of physics, College of Science for Women, University of Baghdad Iraq |
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Polyaniline films were electrochemically synthesized of aniline in a two-electrode cell. It was observed that the doping influences the color of PANI thin films, which passes from blue to dark green color. Characterizations were made using FT-IR (Fourier transform spectroscopy) and a group theory analysis. A complete assignment of the fundamental infrared modes (400-4000 cm-1) of polymer is proposed. Experimental spectra were compared with that calculated by AM1, PM3, RM1, and MNDO. Results indicated that, for aniline, bianiline, and polyaniline, PM3 and RM1 calculated frequencies are in good agreement with experimental data. The geometry and vibrational calculations of four-ring unit (emeraldine base, EB) are believed to be a good representation of polyaniline.
Keywords |
| Polyaniline, Electro-Polymerization, Vibrational modes, Infrared Spectroscopy. |
INTRODUCTION |
| Electrochemical Polymerization is one of the excellent methods to synthesize conducting polymers because their area and thickness can be controlled. Polypyrrole (PPP), Polythiophene (PT) and Polyaniline (PANi) all contain conjugating () electron system i.e. they are members of the same class of polymers, and have been intensively studied from both experimental [1-5] and theoretical [6-8] points of view. Polyaniline is one of the most potentially conducting polymers, due it's easy synthesis, high conductivity, and unique redox properties [9-12]. PANi has attracted attention due to its stability in air in the oxidized state, which can be reversibly doped in aqueous medium from a semi conducting state ( ≈ 10-8 Ω-1cm-1) to a conducting state ( ≈ 102 Ω-1cm-1) [13]. Conducting polyaniline, on account of its excellent optical and electronic properties is promising in many fields, in electronics. During the last years, conducting polymers have conjured great interest in the world of research due to their various physical, chemical properties and their numerous possible applications [14]. Conducting polymer PANi attracted much attention because of its physical and chemical characteristics which make it interesting material for applications in different areas such as Light-weight batteries [15-18], capacitors [19], electrochromic devices[20, 21], photoelectric cell [22, 23], light emitting diodes [24], biosensors [25], sensors [26], switchable membranes [27], anti-corrosive coatings on metals [28]. The microstructure of conductive PANi can vary greatly with the preparing methods and processing conditions. Sazou et al reported polyaniline coating on stainless steel by potentiodynamic and potentiostatic deposition [29]. Zhang et al prepared a series of polyaniline/Carbon nanotube array composite electrodes by cyclic voltammetry electrode position, to improve the capacitive performance of PANi/CNTA Composites [30]. Wu et al recently studied the PANi counter electrode coated on conductive glasses using a chemical synthesis method [31]. The present work is conducted to study the polymerization of aniline by electrochemical method and using attenuated absorption Fourier-transform infrared spectroscopy. The experimental spectra are compared with calculated frequencies spectra using (hyperchem 8 packages) |
II. EXPERIMENTAL |
| An amount of 0.1 and 5x10-2M aniline were added to 0.1M of electrolytic medium consisted of Lithium tetrafluoroborate and acetonitrile, the mixture was placed and stirred in a cell (All chemicals and materials were obtained from Sigma-Aldrich chemical Co.). The solution was degassed by argon bubbling; because the presence of oxygen affects the quality of the prepared conducting polymers. The polymer thin films formed on gold electrode, but other electrode materials such as Pt, SnO2, In2O3 are also suitable for this purpose, when the potential value > 4v the current increases sharply, and stabilized after about three minutes. The Au surface was then covered by a blue, highly adhesive deposit, it's high conductivity allowed a fast growth of the film of many m thickness. The color of the solution was observed during reaction, it was opaque blue at the beginning and it became dark green and viscous at the end of the reaction. The thickness of films can be easily controlled by the Polymerization time as reported in our previous paper [32]. The electrochemical dedoping is carried out by shortening the external circuit of the electrolysis cell to obtain a neutral PANI films, the resulting film contained some traces of dopant. A compensation treatment was needed by immersing it in methanol for about 48 hours followed by drying in vacuum. These procedures resulted in an extremely low conductivity PANi Film less than 10-9 Ω-1cm-1 [33]. This film can be easily doped again by either chemical or electrochemical means, by exposing the film to iodine or bromine gas, and applying appropriate voltage across the electrodes of the electrolytic cell which was then rinsed in acetone to compensate trace amount of dopant. The conductivity of conductive PANi thin films was measured using the standard four-probe technique; the measured conductivity of our films was about 10 Ω-1cm-1. We have also obtained a dark green powder of PANi in the viscous solution, to extract a powder sample we wetted a glass substrate with some of the resulting solution. The solution was let to settle for 24 hours at room temperature and then dried at 60 oC for 10 hours; the powder was then peeled out the glass substrate. |
III. GROUP THEORY ANALYSIS |
| This part presents the calculation of number of modes of chain vibrations for the monomer and polymer. The general formula for Aniline and calculated structure is shown in fig.1. |
The application of group theory to monomer presents C2v symmetry, the calculation shows that we have 32 modes of
vibrations, which are active in both I.R and Raman and 4 modes are Raman active as seen from eqs.1 & 2 respectively
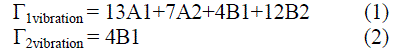 The 36 modes of vibrations of aniline consist of: five Stretching modes (C-H) situated around 3050 to 3078 cm-1, five
deformation modes (C-H) in plane situated around 1300cm-1, and five bending modes out of plane (C-H) situated
around 700 to 1000 cm-1. We also have two stretching modes (N-H) situated around 3250 to 3300 cm-1, two
deformation modes in plane (N-H) situated around 1650 to 1580 cm-1, and two deformation modes out of plane (N-H)
situated around 660 to 900 cm-1. For the vibration of C-N, we have one mode stretch (C-N) situated around 1335 to
1250 cm-1, One mode of bending (C-N) situated around 1000 cm-1, One mode of deformation out of plane (C-H)
situated around 500 cm-1. There are also 12 modes of ring vibrations that consist of four Stretching modes, four bending
and four out of plane vibration modes.
The polymerization of aniline monomer can be found in one of three idealized oxidation states: Leucoemeraldine-white
clear, emeraldine-green or blue, pernigraniline-blue/violet. These three types are different in the degree and nature of
The 36 modes of vibrations of aniline consist of: five Stretching modes (C-H) situated around 3050 to 3078 cm-1, five
deformation modes (C-H) in plane situated around 1300cm-1, and five bending modes out of plane (C-H) situated
around 700 to 1000 cm-1. We also have two stretching modes (N-H) situated around 3250 to 3300 cm-1, two
deformation modes in plane (N-H) situated around 1650 to 1580 cm-1, and two deformation modes out of plane (N-H)
situated around 660 to 900 cm-1. For the vibration of C-N, we have one mode stretch (C-N) situated around 1335 to
1250 cm-1, One mode of bending (C-N) situated around 1000 cm-1, One mode of deformation out of plane (C-H)
situated around 500 cm-1. There are also 12 modes of ring vibrations that consist of four Stretching modes, four bending
and four out of plane vibration modes.
The polymerization of aniline monomer can be found in one of three idealized oxidation states: Leucoemeraldine-white
clear, emeraldine-green or blue, pernigraniline-blue/violet. These three types are different in the degree and nature of |
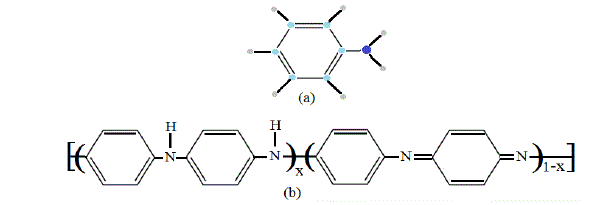 |
| Fig. 1 General Formulas for both aniline (a), and the conductive polyaniline (emeraldine form (b)) |
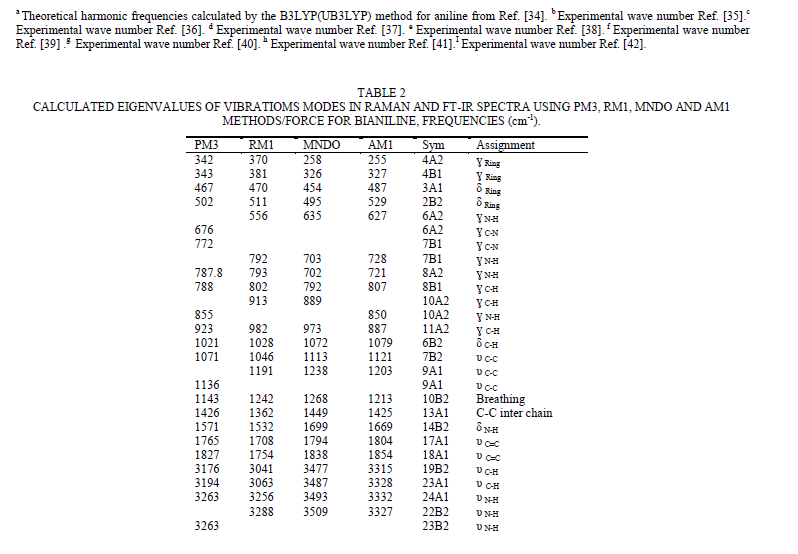
| polymerization. The emeraldine form of PANi is the most symmetric. Emeraldine base (EB) is also regarded as the most useful form of PANi due to its high stability and good electrical conductivity. The bianiline form presents C2v symmetry also; the calculations show that we have 61 modes of vibrations that are active in both I.R and Raman as given in eq.3, while 11 modes are Raman active as given in eq.4. |
 |
 |
 |
IV. RESULTS AND DISCUTION |
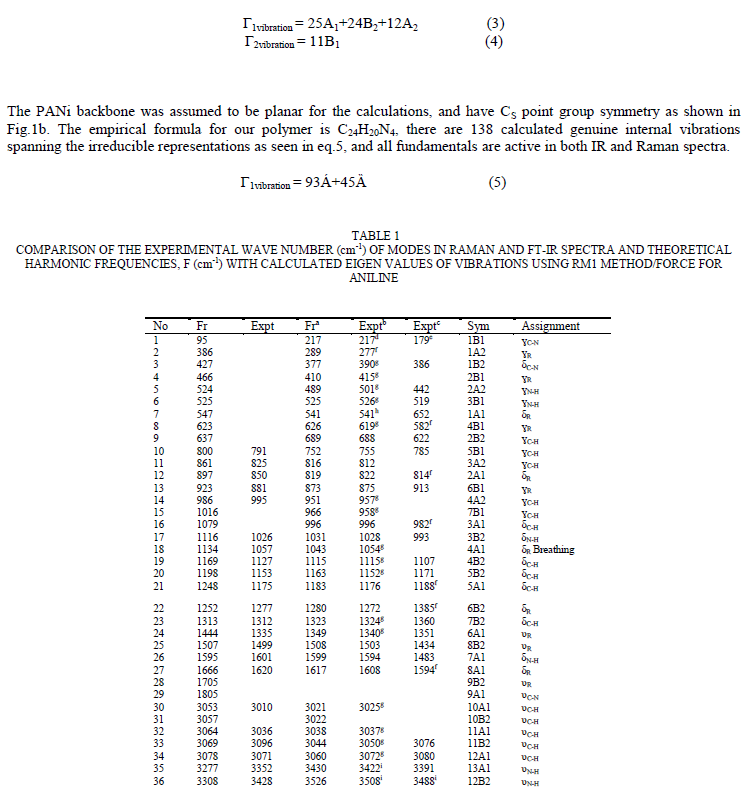
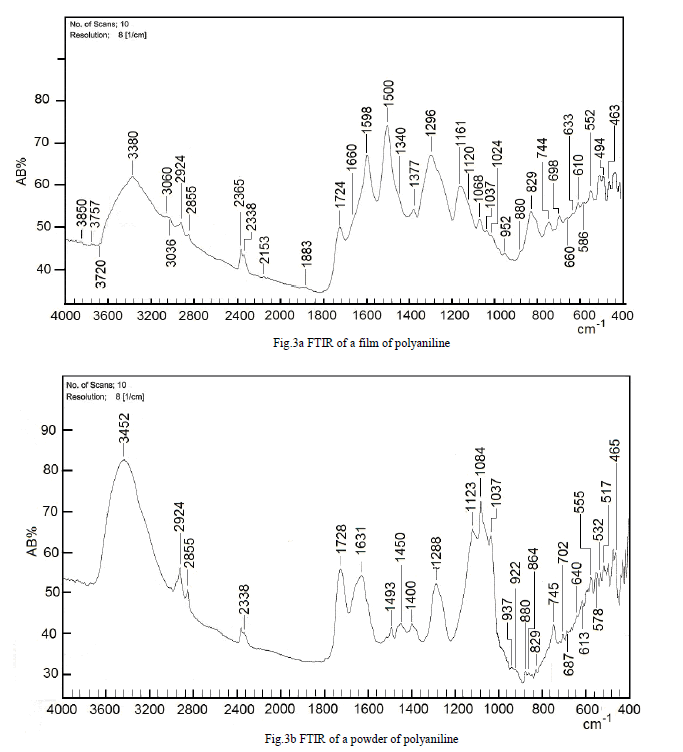
| To study the I.R spectra of both aniline and polyaniline, we need precise assignment of vibrational modes. This is achieved by a comparison of the band positions and intensities observed in I.R spectra with frequencies and intensities from the calculations of the molecular model used in our study of aniline. For this purpose, the hyperchem 8 package employed for theoretical calculations of the vibration modes active in I.R and Raman, this program uses the semiempirical (RM1, PM3, MNDO, AM1) methods for electronic molecular structure modeling. These calculations were performed in order to determine the vibrational and rotational eigenvalues of aniline, bianiline and polyaniline. These calculated values were used to interpret the experimentally obtained of I.R absorption bands. Some frequencies of these modes are listed in tables 1, 2 and 3. The FTIR spectrum of aniline is shown in fig.3 it shows the presence of C-NH2 stretching vibrations at 3352 and 3428 cm-1 [34]. The C-H stretching vibrations of benzene derivatives generally appear above 3000 cm-1. In our I.R Spectrum of the titled compound, the bands at 3071, 3096, 3036, and 3010 cm-1 are assigned as C-H stretching vibrations [43-45]. From the above discussion, this is clear that experimental and theoretical C-H stretching vibrations are nearly in agreement with each other. The three bands the main one at 995 cm-1, and the two weak ones at 791 and 725 cm-1 in fig.3 are C-H out of plane-bending modes [34-36]. The in-plane bending vibrations modes are situated at 1127, 1153, 1175 and 1312 cm-1 [34-36], [42, 46, 47]. For the stretching vibrations of N-H in aniline, we have the bands at 3352 and 3428 cm-1 [34-36, 42]. The package of bands 1693, 1776, 1800, 1933, 2380, 2653 and 2785 cm-1 came from overtone combinations [45]. The ring carbon-carbon stretching vibrations occur at 1620, 1601 and 1466 cm-1 and assigned to C-C stretching vibrations. Our data shows good agreement with Varsanyi [48]. A close examination of fig.4a, b reveals that the spectra contain all the main characteristics of polyaniline bands i.e. the vibration frequencies of the broad band at 3400 cm-1 fig.4a is attributed to the characteristic free N-H stretching vibration which indicates the presence of secondary amino groups (-NH-) [49]. The weak bands at 2924 and 3036 cm-1 arise from the aromatic C-H stretching vibrations. The spectrum also exhibits main bands at 1500 and 1567cm-1 corresponding to the benzenoid (C=N) and quinoid (C=C) rings stretching frequencies respectively [50-53, 47]. These bands are very week in the case of polyaniline powder as shown in figure 4b. The absorption band at 1293cm-1 fig.4a is related to C-N stretching vibration of secondary aromatic amine [54-56], this band shifted to 1288cm-1 as shown in fig.4b. The band, which appeared at 1161cm-1 in fig. 4a is assigned to the rings 1, 2, 3, 4 of PANI film [53]. In the case of Polyaniline powder, this band disappeared as shown in fig.4b. The 1120 cm-1 band can be assigned to the vibration of C-H mode, which is attributed to protonation [57-62], the broadness of this peak is owing to the high degree of electron delocalization [63], which is expected because of the greater degree of oxidation. The two bands at 1037 and 952 cm-1 represent the aromatic ring deformation and quinoid ring plane deformation respectively [64]. The C-H out of plane bending vibration band of 1, 4- distributed benzene rings appears at 744 cm-1[65]. |
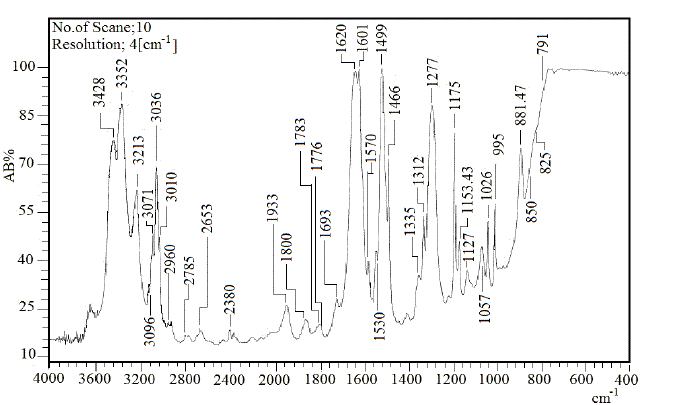 |
| Fig.2 FTIR of Aniline |
 |
V. CONCLUSION |
| Thin films of PANI conducting polymer can be successfully synthesized by electrochemical polymerization of aniline. FTIR spectroscopy is a powerful tool to investigate the spectral properties of these polymers, from these investigations we hope to obtain a better understanding these highly conducting polymer materials. Our results have been consolidated from vibrational bands spectrum analysis, which showed that most of these bands are attributed to CN, C-C, and C-H stretching, in plane bending and out of plain vibrations as well as N-H modes of vibrations. From the comparison of both experimental and calculated frequencies, it is concluded that the results using PM3 and RM1 calculations are more accurate than those obtained from MNDO and AM1, these calculated frequencies are in good agreement with experimental frequencies for both monomer and polymer. The characteristics of PANI films have been examined with respect to the samples prepared with different concentrations of monomer and different applied voltage, it is probably that the electronic properties of PANI films prepared with higher concentrations of monomer and higher applied voltage are poor compared with those prepared at lower monomer concentration and applied voltage. From comparison of PANI powder spectra with those of thin films, we conclude that the frequency bands intensities, which characterize the PANI powder in the region between 1400 and 1500cm-1 are lower than in the thin film case; this can be used as an indicator of a lower degree of polymerization. |
ACKNOWLEDGEMENTS |
| The author thanks the college of science for women, university of Baghdad for financial support. |
References |
|