ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Aisha M. Al-Turkustani1 and Khadijah M. Emran2
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Corrosion of aluminum in HCl solutions and its inhibition by aqueous extract of Olive seeds (OS) extract have been reported. The study was carried out using weight loss, hydrogen evolution at 30 and 40°C and the results were supporting by surface examination study (SES) and electrochemical impedance spectroscopy (EIS) measurements at 30°C. Experimental results indicated that the corrosion of aluminum increased with increasing acid concentration and rising temperature. The presence of OS extract inhibit the general and pitting corrosion of aluminum in HCl solutions. This effect was remarkably increased by increasing the concentration of OS extract. The inhibition efficiency increased with rising temperature in the presence of inhibitor, especially at low concentrations. The adsorption of the inhibitor was also found to be spontaneous, chemical and consistent with the assumptions of Langmuir adsorption isotherm.
Keywords |
| Aluminum corrosion, Corrosion inhibition, Olive seeds, hydrochloric acid solutions. |
INTRODUCTION |
| Aluminum is an important metal in industry owing to its many excellent characteristics including good electrical and thermal conductivities, low density, high ductility, and good corrosion resistance. It is widely used as a material for automobiles, aviation, household appliances, containers, and electronic devices, which get corroded easily in the presence of acids[1,2] such as hydrochloric (HCl), sulfuric (H2SO4) and phosphoric (H3PO4) acid, ect. |
| Hydrochloric acid is one of the most widely used agents in the industrial sector. Due to the aggressiveness of acid solution to aluminum, the use of inhibitor to prevent the metal dissolution process will be inevitable[3]. The protection of aluminum from the corrosive chloride attack has been studied by many investigators using organic and inorganic compounds as corrosion inhibitors[1,2,4-12]. It has been reported[13] that the inhibitor molecules are bonded to the metal surface by chemisorption, physisorption, or complexation with the polar groups acting as the reactive centers in the molecules. |
| Most synthetic inhibitors are highly toxic and thus lead to the investigations on the use of a naturally occurring corrosion inhibitor which at the same time is not harmful to both human and the environment[14]. |
| Olive stone and seeds are an important product generated in the olive oil extraction and pitted table olive industries. As a lignocelluloses material, the hemicelluloses, cellulose, fatty acids, vitamins, lignin and other water soluble components are the main components of olive seeds as wells as protein, fat, phenols, free sugars composition. Its primary fatty acids are oleic and linolenic acids. Oleic acid is monounsaturated and makes up about 55%-85% while linoleic acid is polyunsaturated and makes up about 9% of the oil. The main use of this biomass is as combustion to produce electric energy or heat. Other uses such as activated carbon, furfural production, plastic filled, abrasive and cosmetic or other potential uses such as biosorbent, animal feed or resin formation have been cited[15]. |
| The objective of the present work is to report the evaluating of behavior of aluminum corrosion and the effect of aqueous extract of Olive seeds as anticorrosion for aluminum in 1M hydrochloric solution at different temperatures using chemical (ML& HE), electrochemical (EIS) and surface examination study (SES). |
MATERIALS AND METHOD |
| The corrosion behavior of OS extract on aluminum was determined via gas-volumetric (hydrogen evolution (HE)) and gravimetric (mass loss (ML)) measurements. The adsorption nature and surface morphology analysis using scanning electron microscope (SEM) were also determined. |
| 1- PREPARATION OF OLIVE SEEDS EXTRACT |
| Olive seeds were collected and washed under the running tap water and then were dried for three days to remove water before being ground into fine powder. Initially, the dried powder was diluted with deionized water in the ratio of 1:10(w/v). The solution was then placed in a hot water for 24h and then filtered to obtain the stock solution. |
| 2-SPECIMEN PREPARATION |
| Aluminum specimen having the composition (wt%) of 0.08 C,0.01 Si, 1.26 Mn, 0.02 P and remaining Al was used. The sample with 5x1 cm2 dimensions was used. |
| 3- SOLUTIONS PREPARATION |
| About 1 M HCl solution was prepared by the dilution of 37% HCl using deionized water. The concentration range of Olive seeds extract employed was varied from 1 to 10%v/v. |
| 4- GRAVIMETRIC (ML) MEASUREMENTS |
| The aluminum specimen was immersed in different concentrations of HCl. For inhibition study, the mass loss of aluminum specimen was determined after 1h of immersion in presence of different concentrations of Olive seeds extract in 1M HCl at 30°C and 40ºC. Then the specimen was washed, dried and weighed. The percentage inhibition efficiency by Gravimetric (ML) measurements (Inh.ML%) was calculated using the following formula: |
 |
RESULTS AND DISCUSSION |
| 1-EFFECT OF HCL CONCENTRATION |
| The corrosion rates of aluminum in different concentrations of HCl solution (0.25-1.5M) was discussed by monitoring the weight loss and the volume of hydrogen gas evolved at fixed time. Figure 1 shows representative plots of the volume of hydrogen gas evolved as a function of time in different concentrations of HCl solution at 30o C. Table 1 shows the corrosion rates obtained from the slope of the linear part of the gasometry plots for the test solutions and corrosion rates obtained from the mass loss technique. The corrosion is attributed to the presence of water, air and H+, which accelerate the corrosion process. It is clear that the corrosion of the aluminum in HCl increases with the concentration of the acid. This observation is attributed to the fact that the rate of chemical reaction increases with increasing acid concentration. |
| 1-EFFECT OF TEMPERATURE ON THE CORROSION OF ALUMINUM |
| There is a progressive increase in weight loss of aluminum and hydrogen evolved in 1M HCl as the temperature is increased from 30°C to 60°C as shown in Fig. 2. This signifies that the dissolution of the metal increased at higher temperatures. This observation is attributed to the general rule guiding the rate of chemical reaction, which reveal that chemical reaction increases with increasing temperatures. Also, increased temperature favors the formation of activated molecules, which may be doubled in number, with 10ºC rise in temperature, thereby increasing the reaction rate. This is because the reactant molecules gain more energy and are able to overcome the energy barrier more rapidly [17,18]. An increase in temperature may also increase the solubility of the protective films on the metals, thus increasing the susceptibility of the metal to corrosion[19,20]. The solubility of oxygen gas decreases with increase in temperature. Thus oxygen concentration is expected to be more at higher temperature which in this case is higher at 60°C than at 50°C, 40°C and 30°C. The presence of high concentration of oxygen thereby causes the metal to corrode faster. Also the protective film which is solid becomes more soluble as the temperature is increased. |
| 2-INHIBITION ACTION OF OLIVE SEEDS EXTRACT ON THE CORROSION OF ALUMINUM |
| Figures 3&4 represent plots of hydrogen gas evolved for aluminum corrosion in the presence of different concentrations of inhibitor (OS) at 30oC and 40oC, respectively. It is observed from the figures that the volume of hydrogen gas evolved varies linearly with time and the slope of the liners were less in the presence of OS extract. The general decrease in hydrogen gas evolution with time as concentration of additives increased from 1%v/v to 10%v/v confirms that the presence of the additives in 1M HCl solution reduced the corrosion of aluminum. The hydrogen evolution technique enables us to assess the inhibitory effect of the inhibitor at 1M HCl corrodent concentrations, this means that the corrosion rate of aluminum was decreased with increasing the concentration of OS extract compared to the free acid solution at 30°C & 40°C. |
| Calculated values of the corrosion rate and inhibition efficiency (Inh. %) for the different systems from HE and ML in the presence of OS extract compared to the blank solution are presented in Tables 1-4. The data presented in the Tables follow the same trend observed for two methods HE and ML. Inhibition efficiency is also observed to generally increase with rising temperature, suggesting that metal/inhibitor interactions become high at low concentrations of OS extract (1,3 and 5%v/v), while at 10%v/v, the Inh.% at 40°C is lower than that at 30°C. This means that at low concentrations of OS, chemical adsorption will be occur. At high concentrations of OS the adsorption is physical. |
| 3-SCANNING ELECTRON STUDY (SES) |
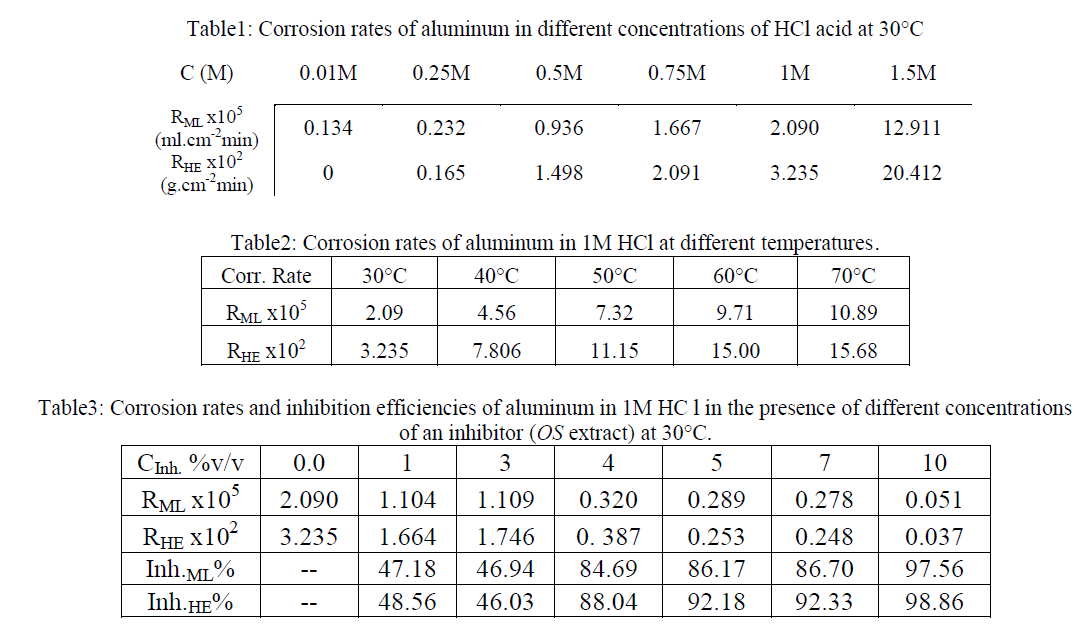
| The surface morphology of aluminum surface after 1hour immersion in the test solution at 30°C in the absence and presence of lower and higher concentration of OS has been done as in Figs.5 (a,b,c). Figure 5(a) illustrates the effect of 1M HCl on the aluminum surface, it appears the general corrosion and pitting corrosion. The pits are deep and contain a local cell. Pitting corrosion may increase the corrosion rate. |
| In Fig .5(b),the presence of thin porous and protective layer on aluminum surface contain of numerous pits less than that appears in case of HCl alone was observed in presence of 3% v/v of OS extract in 1M HCl. It obvious that Inh.ML% is about 46.94% in this case. On comparing Fig. 5 (c), which illustrates the effect of 10% v/v of OS extract, Inh.ML% is about 97.56. It appears that the disappearance of pits and the formation of an adsorbed film on aluminum surface due to adsorption of Olive Seeds components lead to high corrosion decrease at this concentration. This confirms the results obtained from mass loss and hydrogen evolution studies. |
| 4-ELECTROCHEMICAL IMPEDANCE SPECTROSCOPIC (EIS) STUDIES |
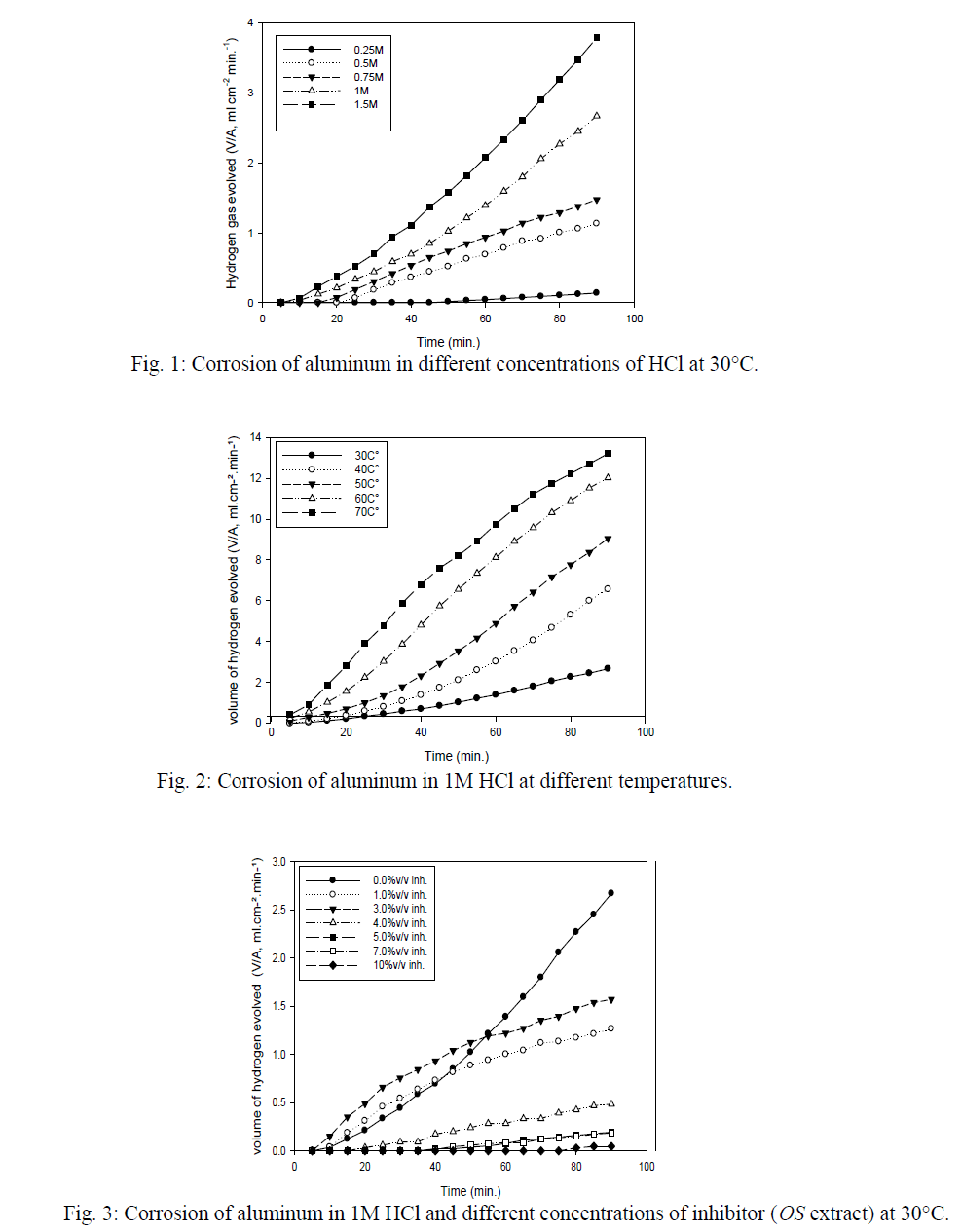
| The corrosion behavior of aluminum in 1 M HCl in the presence of 3%v/v and 10%v/v was investigated by the EIS method at 30°C after immersion for 1 h. The Nyquist plots of aluminum in 1 M HCl in absence and presence of OS, 10 & 3%v/v are shown in Fig.6. The Nyquist plots are significantly changed on addition of inhibitor. The impedance of the inhibited system increased with inhibitor concentration. The results were recorded in Table 5, and it clear that this result is supports the previous results. |
| 4- ADSORPTION BEHAVIOR OF OS ON ALUMINUM SURFACE IN HCL ACID SOLUTION |
| The adsorption process depends on the structure of the inhibitor (extract molecules), the nature and charge of metal surface, temperature, the type of electrolyte solution and the number of active centres on the surface. The adsorption process can be described by two main types of interactions: physical adsorption and chemical adsorption, or both[21,22]. |
| The decrease in corrosion rate of aluminum sample by adding the Olive Seeds may be due to the adsorption of components of the extract on the aluminum surface or to form a protective barrier layer which separate the aluminum surface from the center of corrosion[23,24]. |
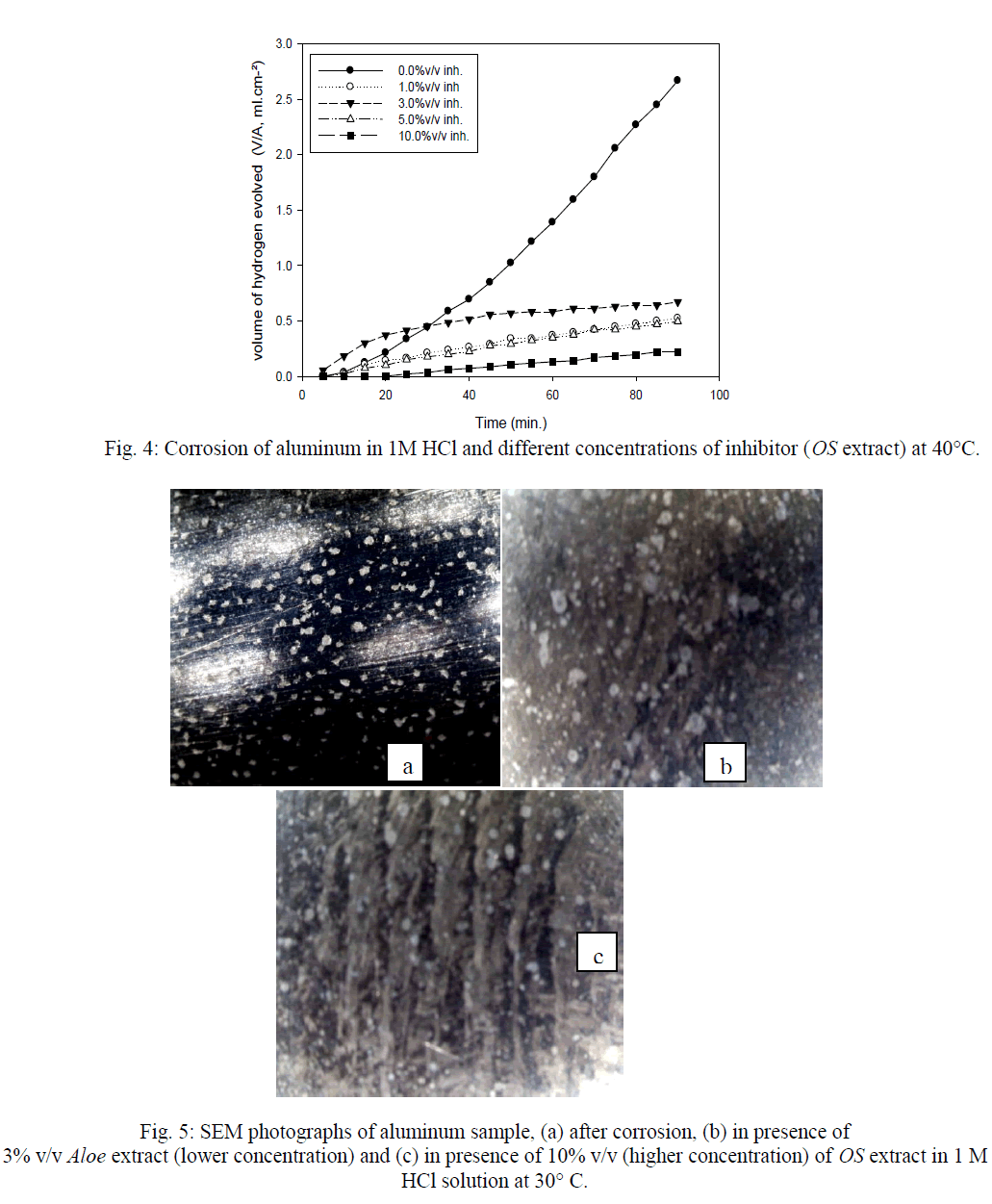
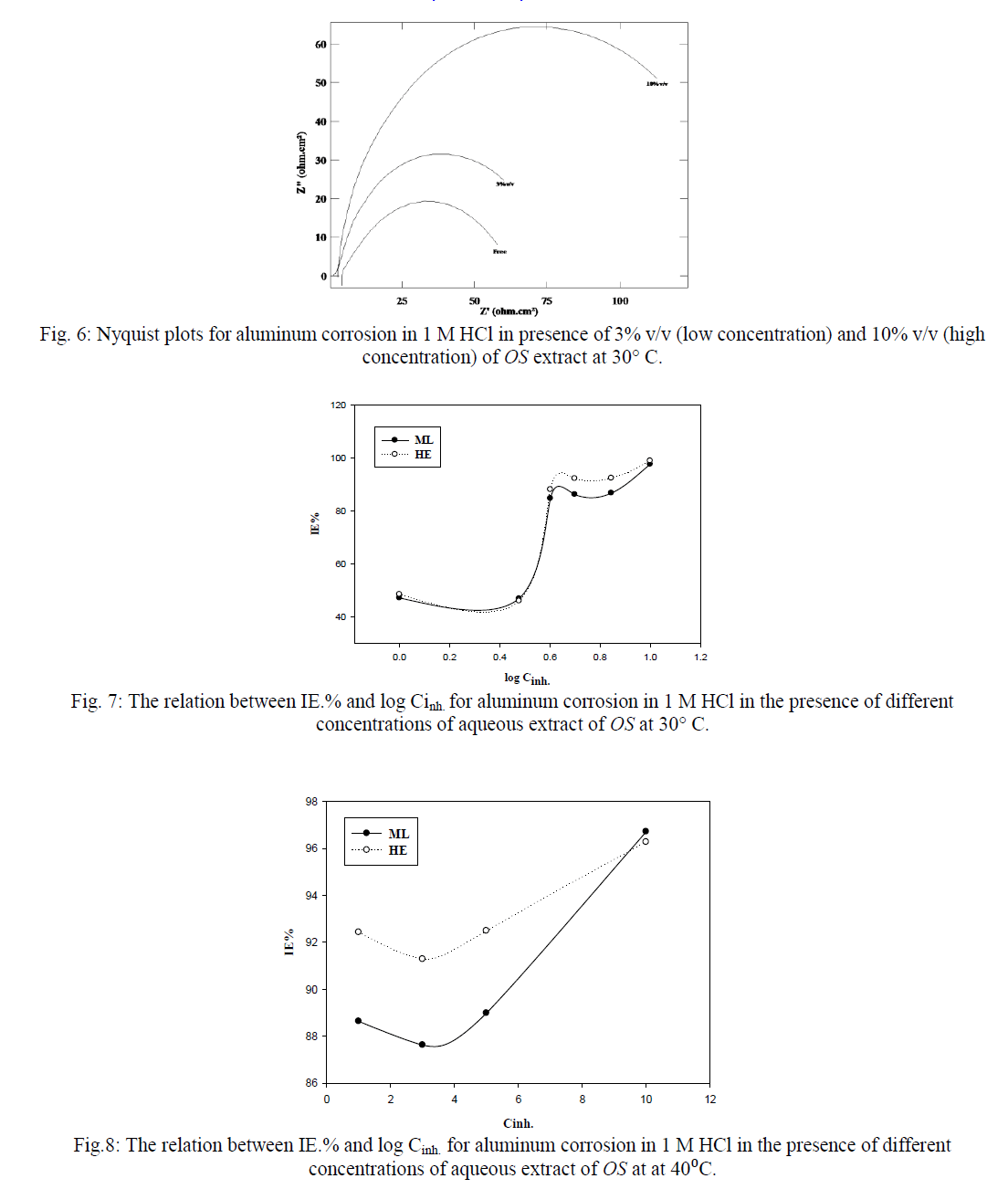
| Figures (7&8) illustrate the relationship between Inh.% deduced from different methods and the logarithm of the concentration of OS extract at both 30°C and 40°C, respectively. Figure 7 shows an adsorption curves with S-shape adsorption isotherm at 30°C, which indicates that the interaction takes place in one step attributed to the formation of a single layer of extract molecules adsorbed on aluminum surface. At 40°C the curve is extremely S-shape but not perfect, Fig. 8. |
 |
| By examining the chemical composition of Olive seeds, shows that the extract contains compounds such as protein, phenols, free sugars and fatty acids, oleic and linolenic acids. In view of these components it is clear that the majority of organic compounds containing oxygen atom and all have pairs of free electrons. The inhibitive property of OS may be explained by considering the adsorption of the OS extract molecule through the free electrons on the oxygen atom in these compounds and complex formation (surface chelation) on the corroding metal surface. These may be responsible for the formation of an oriented film layer, which essentially blocks discharge of H+ and consequent dissolution of the metal ions and reduced the interaction between the aluminum with central corrosion and thus decrease the corrosion rate. |
CONCLUSION |
| The effect of acid concentration and the effect of addition the aqueous extract of Olive seeds (OS) on the corrosion of aluminum has been studied at 30 and 40°C. The following conclusions may be drawn: |
| 1) The chemical (HE and ML) results showed that the corrosion rate of aluminum sample is increase with increasing acid concentrations (0.25-1.5) M and rising temperature (30-40)°C. |
| 2) The aqueous extract of OS acts as good inhibitor for the corrosion of aluminum metal in 1 M HCl solution. |
| 3) At 40°C, increasing of inhibition is found as concentration of the OS increased and Inh. % is higher than at 30°C. |
| 4) The adsorption of OS extract molecules on aluminum metal surface follows Langmuir adsorption isotherm, the values of Kads., Ea indicate chemical adsorption. |
| 5) It appears from SES and EIS methods that the presence of OS extract an adsorbed film on aluminum surface was contained due to adsorption of Olive Seeds components on aluminum surface which lead to corrosion inhibition. This results confirms with that obtained from mass loss and hydrogen evolution studies. |
 |
 |
 |
 |
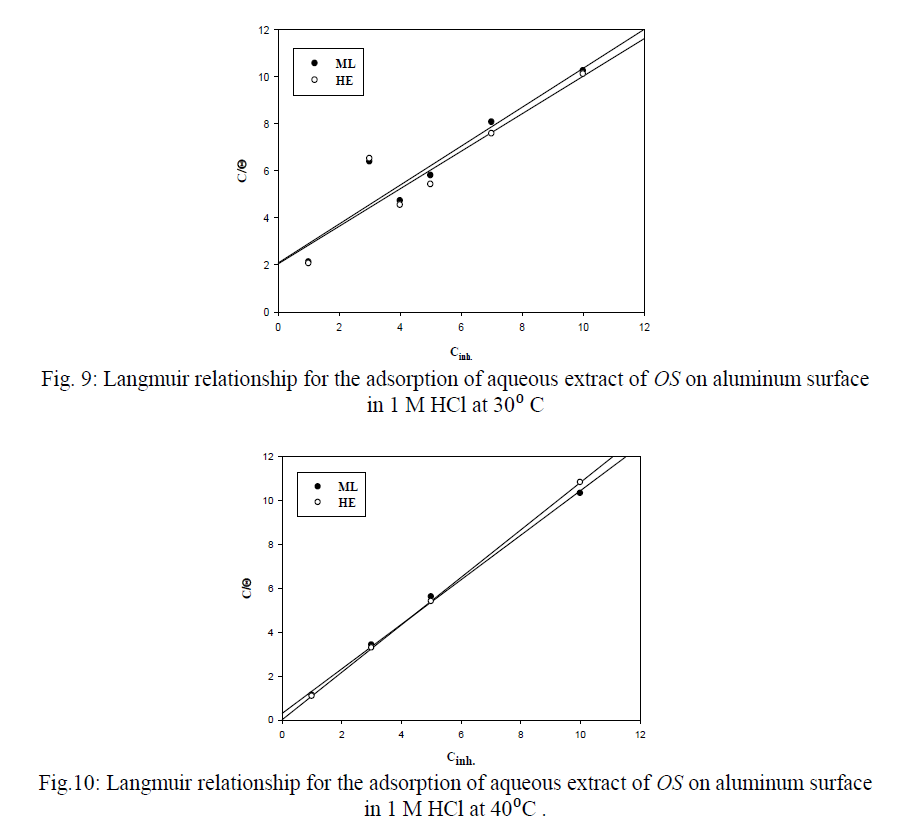 |
References |
|