Key words
|
| Lamivudine, Hepatitis B, HIV infections, Carbopol, Hydroxy propyl methylcellulose, xanthan gum |
Introduction
|
| Lamivudine (2(1H)-Pyrimidinone, 4-amino-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl]-,(2R-cis)-. (–)-1-[(2R, 5S) -2-(Hydroxymethyl)-1,3-oxathiolan-5-yl]cytosine.) is a white to off-white crystalline solid with a solubility of approximately 70 mg/mL in water at 20°C. Lamivudine is a |
antiviral drug used in the treatment of hepatitis B and HIV infections [1 - 2]. |
| It has a shorter half-life of 2-6 hrs, oral availability is 86% and it is eliminated rapidly from the plasma compartment within few hours. Prescribed dose is 300 mg daily, administered as either 150 mg twice daily or 300 mg once daily. Lactic acidosis and severe hepatomegaly with steatosis [3], Hepatic decompensation in patients co-infected with HIV-1 and Hepatitis C, Pancreatitis are side effect of Lamivudine [4]. |
MUCOADHESIVE DRUG DELIVERY SYSTEMS
|
| Mucoadhesive medication conveyance frameworks delay the residency time of the measurement structure at the site of use or assimilation and encourage a close contact of the dose structure with the fundamental ingestion surface and along these lines add to enhanced and/or better remedial execution of medications. The potential site for connection of any bioadhesive framework or mucoadhesive frameworks incorporates buccal, oral, vaginal, rectal, nasal and visual destinations as these contain the mucosal covering layer [5, 6]. The term bioadhesion alludes to any security framed between two organic surfaces or a security between a natural and an orderly surface. On account of bio glue drug conveyance frameworks, the term bioadhesion is commonly used to portray the grip between polymers, either manufactured or characteristic, and delicate tissue (i.e., gastrointestinal mucosa). In spite of the fact that the objective of numerous bio glue conveyance frameworks may be a delicate tissue cell layer (i.e., epithelial cells), the real cement bond may frame with either the cell layer, or a mucous layer, or a mix of the two [7 - 10]. |
| In occasions in which bonds shape in the middle of bodily fluid and polymer, the term mucoadhesion is utilized synonymously with bioadhesion. As a rule, bioadhesion is a comprehensive term used to portray cement associations with any organic or naturally inferred substance, and mucoadhesion is utilized just when depicting a bond including bodily fluid or a mucosal surface [8, 9, 2 - 4]. |
MECHANISMS OF BIOADHESION
|
| The mechanisms responsible for the formation of boiadhesive bonds are not completely clear. In order to develop ideal bioadhesive drug delivery systems, it is important to describe and understand the forces that are responsible for adhesive bond formation [10 - 15]. Most research has focused on analyzing bioadhesive interactions between polymer hydro gels and soft tissue [16]. The process involved in the formation of such bioadhesive bonds has been described in three steps: |
| 1. Wetting and swelling of polymer to permit intimate contact with biological tissue, |
| 2. Interpenetration of bioadhesive polymer chains and entanglement of polymer and mucin chains, |
| 3. Formation of week chemical bonds between entangled chains. |
MUCOADHESIVE POLYMERS
|
| Mucoadhesive polymers are water insoluble and water dissolvable polymers which are swellable systems joined by cross- connecting specialists [17 - 19]. The polymer ought to have ideal extremity to verify that it is adequately wetted by the bodily fluid and ideal smoothness that allows the shared adsorption and interpenetration of polymer and bodily fluid to occur. A perfect polymer for a mucoadhesive medication conveyance framework ought to have non dangerous [20], non-aggravation and nonabsorbable from the gastrointestinal tract. What's more, it ought to stick rapidly to wet tissue and ought to have some site specificity. The polymer ought to permit simple consolidation of the medication [21] and must not decay on capacity or amid rack life of the dose structure [22 - 29]. What's more, it ought to ideally shape a solid non covalent bond with the mucin epithelial cell surfaces. |
METHODS
|
Construction of Standard Curve of Lamivudine
|
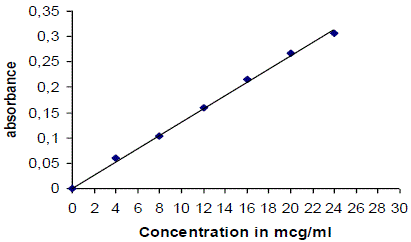
| lamivudine can be estimated spectrometrically at 270 nm as it obeys beer- lambert’s law. Stock solution is 100mg of lamivudine was dissolved in 100ml of 0.1 n hcl, so as to get a solution 1000 μg/ml concentration [30, 31]. |
Standard solution
|
| 10ml of stock solution was made to 100ml with 0.1 N Hcl thus giving a concentration of 100 μg/ml. Aliquot of standard drug solution ranging from 4ml to 24 ml were transferred in to 10ml volumetric flask and were diluted up to the mark with 0.1 N Hcl. Thus the final concentration ranges from 4-24 μg/ml. Absorbance of each solution was measured at 270 nm against 0.1 N Hcl as a blank. A plot of concentrations of drug versus absorbance was plotted. |
COMPATIBILITY STUDY
|
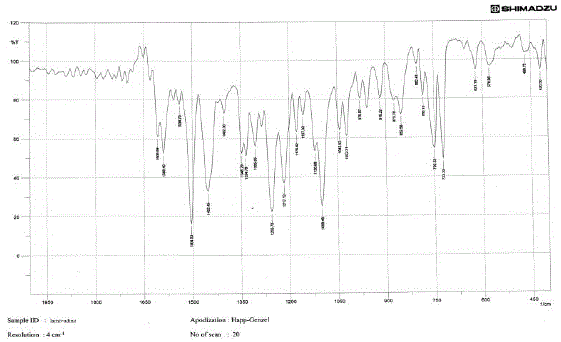
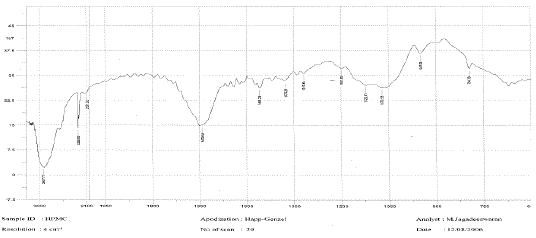
The medication was distinguished and affirmed by FTIR range. Fig 02 demonstrated the IR range of
lamivudine. Fig 03 and Fig 04 demonstrates the IR range of the polymer Ethylcellulose and Hydroxy propyl
methylcellulose separately. Fig 03 demonstrates the IR range of the physical blend of Carbopol, hydroxy
propyl methylcellulose, xanthan gum and the medication lamivudine [28-31]. From the Fig 13 it was inferred
that the medication alongside the polymer demonstrated no adjustment in any trademark crest of the
medication, which affirms that there is no collaboration between the medication and the polymer [31 - 37]. |
PREPARATION OF TABLETS
|
| Mucoadhesive l tablets containing lamivudine were arranged by direct pressure system. The elements of the center layer (Table No: 02) were measured precisely and blended by trituration in a glass mortor & pestle [37 - 42]. The blend was then packed utilizing 8mm bite the dust by a tablet press. To acquire consistent tablet weight the splash dried lactose was included as filler excipient in the center layer. After pressure of tablet the upper punch was uprooted deliberately without aggravating the set up and blended elements of the support layer were included over the tablet and packed once more [42 - 50]. |
Flow Property
|
| The stream property was dictated by measuring the edge of rest. With a specific end goal to focus the stream property, the point of repose(s) was dead set. It is the most extreme point that can be acquired between [51 - 55] the unsupported surface of a powder stack and the flat plane. Estimations of are seldom under 20, and estimations of up to 40o show sensible stream potential. Over 50ºC, then again, the powder streams just with trouble if by any means [56 - 65]. |
| θ = tan-1 (h/r) |
| Where h = tallness the heap, r = range of the heap, θ = point of rest |
| The specimen was taken in a pipe, which settled in a holder (5cm) over the surface at a suitable stature and a chart of sheet was set beneath the channel. The specimen was gone through the pipe gradually [66]. The tallness of the powder load shaped was measured. The perimeter shaped was drawn with a pencil on the chart paper [67 - 70]. The span was measured and the point of rest was dead set. This was rehashed three times for a sample [71 - 76]. |
Determination of bulk density and tapped density
|
| The powder (W) was precisely filled the graduated measuring barrel and the volume (VO) was measured [77 - 82]. At that point the graduated barrel was shut with cover and tapped 100 times and after that, the volume (Vf) was measured and proceeded with operation till the two successive readings were approach [83 - 90]. The mass thickness, and tapped thickness were computed utilizing the accompanying recipes |
| Mass thickness = W/ VO, Tapped thickness = W/ Vf |
| Where, W = weight of the powder, VO = introductory volume, Vf = last volume. |
Compressibility index (Carr’s index)
|
| Compressibility file is an essential measure that can be gotten from the mass and tapped densities. In principle, the less compressible a material the more stream capable it is. A material having estimations of under 20 to 30% is characterized as the free streaming material [91 - 96, 60]. |
| CI = 100 (VO – Vf) |
EVALUATION OF TABLETS
|
Friability test
|
| Friability of the tablets was tried utilizing a Roche friabilator. The heaviness of 10 tablets was noted at first (W1) and set in the friabilator for 4min/100rpm. The tablets were reweighed and noted as (W2). The distinction in the weight is noted and communicated as rate [97 – 104, 62]. |
| Rate friability = (W1-W2) 100/W1 |
Weight variety test
|
| Twenty tablets were chosen indiscriminately and the normal weight was resolved [63 - 65]. Not more than two of the individual weights digress from the normal weight by more than the rate deviation indicated in table and none strays by more than double the rate. IP authority cutoff points of rate deviation of tablet are introduced in the I.P [105 - 111, 68]. |
Medication content consistency
|
| Focus the substance of dynamic ingredient(s) in each of 10 tablets taken aimlessly utilizing the system given as a part of the monograph or by some other suitable scientific strategy [111 - 119]. The tablets conform to the test if not more than one of the individual values accordingly acquire is outside the limits 85 to 115% of the normal quality and none is outside the limits 75 to 125% of the normal worth [120 - 123]. |
| Take the readied Lamivudine tablet and separate the two layers (center and support). Take tablets and pulverized in mortar to fine powder [124-129]. At that point disintegrate the aggregate powder in 100ml phosphate cradle (pH 6.8). The arrangement was suitably weakened with pH 6.8-phosphate cushion. Examine at 270nm in uv–vis spectrophotometry [130 - 136]. |
Water ingestion examines
|
| The water engrossing limit of tables was controlled by gravimetry. The swelling rate of the bioadhesive tablets was assessed by utilizing 1% agar gel plate. The normal weight of the tablet was computed (w1) [137 - 139]. The tablets were set on gel surface in a petridish put in a hatchery at 37±1ºC. Tablets was evacuated at distinctive time interims (0.5, 1.0, 2.0, 3.0, 4.0, 5.0, 6.0, 7.0, 8.0 h) [140, 141], wiped with channel paper and reweighed (w2) [142 - 150]. The swelling record was figured by the equation Swelling list = (w2-w1)/ w |
Estimation of surface pH
|
| The tablets were permitted to swell by keeping them in contact with 1ml of refined water (pH 6.8±0.05) for 2hrs and pH was noted by acquiring the anode contact with the surface of the definitions and permitting it to equilibrate for 1min [151 - 159]. |
In vitro Dissolution studies
|
| Dissolution done by utilizing USP XXIII paddle method, Tablet was keep initial 2 hrs in 0.1 n Hcl and after that phosphate buffer [159 - 164]. |
RESULTS AND DISCUSSION
|
Evaluation of blend characteristics of lamivudine
|
 |
 |
 |
 |
 |
| |
 |
 |
 |
 |
| |
DISCUSSION
|
| In the present work endeavors have been made to create mucoadhesive tablets of lamivudine utilizing direct pressure system including mucoadhesive polymers like Carbopol, different cellulose ethers having diverse level of dissolvability and swellability, for example, Hydroxy propyl methyl cellulose and xanthan gum. Magnesium stearate was incorporated as against disciple [165 - 169]. |
| The FTIR ghastly investigation demonstrated that there was no appearance or vanishing of any trademark top, which affirms the nonattendance of concoction connection in the middle of medication and polymers. |
| The mix physical qualities were considered. The points of rest of all plan mixes F1 to F6were in the reach 31º36' ± 0.825 to 34º35' ± 0.459. The mass thickness, tapped thickness, Corr's record and Hausner proportion were found in the scope of 0.317-0.433gm/cc, 0.43-0.52gm/cc, 13.33-17.18 and 1.15-1.2 separately. It uncovers that all the plan mixes were having great stream attributes and stream rates [170]. |
| Thickness of all details F1-F6were in the scope of 3.20 ± 0.142 to 3.50 ± 0.147 mm [171]. Hardness of all definitions F1-F8 were in the scope of 5.2 ± 0.447 to 5.8 ± 0.447 kg/sq.cm. Rate friability of all details F1 to F8 was in the scope of 0.353 to 0.467 %. |
| The Percentage weight variety for of all definitions F1 to F8 was in the scope of 1.3 to 4.7%. Rate of medication substance for all details F1 to F8 was in the scope of 96.1 ± 1.31 to 99.4 |
CONCLUSION
|
| Mucoadhesion is a subject of current enthusiasm for the configuration of medication conveyance frameworks. Mucoadhesive medication conveyance frameworks are conveyance frameworks which get to be glue on hydration to the mucin layer of a mucosal tissue. These frameworks delay the home time of the measurements structure at the site of use or retention and encourage a cozy contact of the dose structure with the hidden ingestion surface and in this manner add to enhanced bioavailability and better helpful execution of medications. |
| In the present work mucoadhesive tablets of lamivudine were defined utilizing Carbopol, Hpmc and xanthan gum as polymer materials with other basic added substances in tablets and the tablets were assessed for medication discharge and in vitro Mucoadhesive properties. Oral controlled discharge plans of lamivudine were planned and were assessed. The accompanying conclusions are drawn from the outcomes got. |
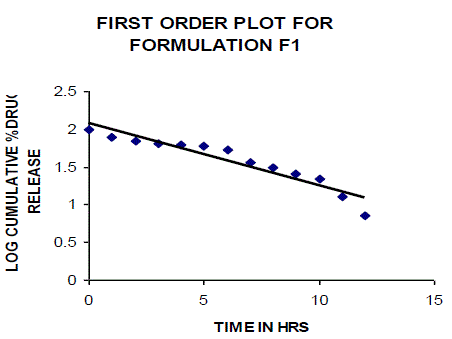
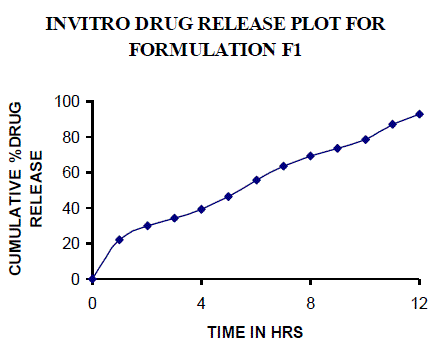
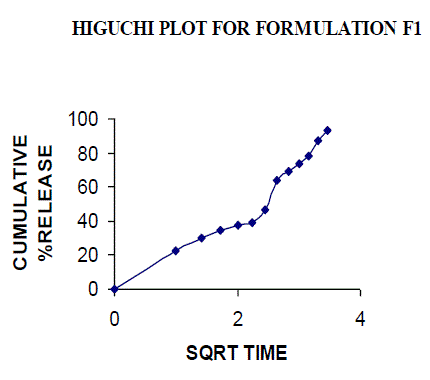
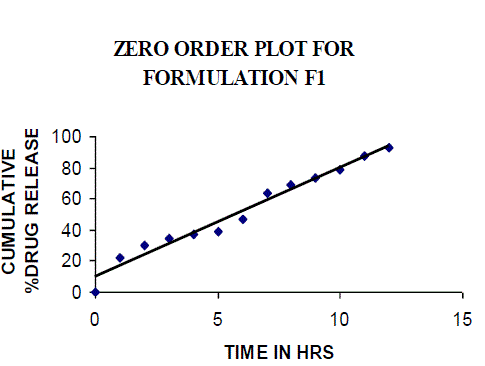
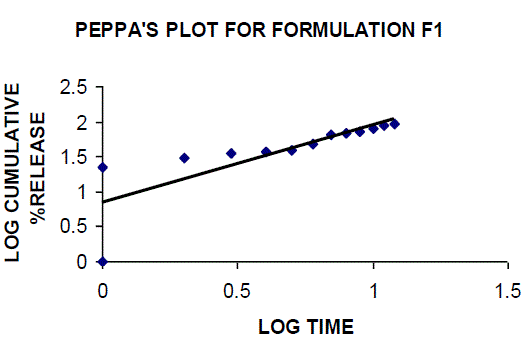
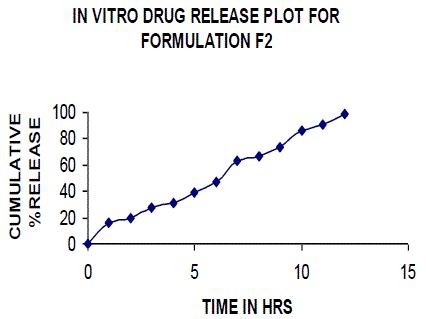
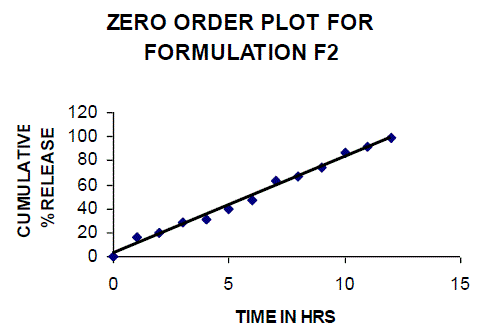
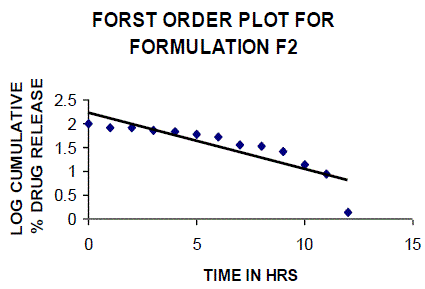
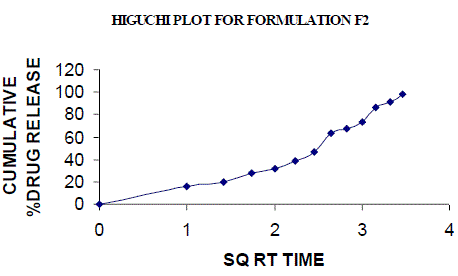
| All the clusters of lamivudine tablets arranged utilizing CARBOPOL, HPMC and XANTHAN GUM were of good quality as to weight varation, hardness, friability, water assimilation studies, surface pH, medication content, invitro medication discharge. All the plans displayed odd (non-ficikan transport) dispersion component and take after zero request active. The plan F6 (CP -15mg, HPMC -40mg, and Mag.Ste-20mg) was chosen as improved detailing taking into account t90% estimation of 10.67 hrs with 99.74 ± 0.06% of medication discharge at 12th hr and had great bioadhesion quality. The mucoadhesive tablets of lamivudine may be a decent approach to sidestep the far reaching hepatic first-pass digestion system and to enhance the bioavailability of lamivudine through mucosa |
Tables at a glance
|
 |
 |
 |
 |
| Table 1 |
Table 2 |
Table 3 |
Table 4 |
 |
 |
 |
 |
| Table 5 |
Table 6 |
Table 7 |
Table 8 |
 |
 |
 |
 |
| Table 9 |
Table 10 |
Table 11 |
Table 12 |
 |
 |
 |
 |
| Table 13 |
Table 14 |
Table 15 |
Table 16 |
|
| |
Figures at a glance
|
 |
 |
 |
 |
| Figure 1 |
Figure 2 |
Figure 3 |
Figure 4 |
Figure 5 |
|
| |
References
|
- Wael Talaat. Micellar Liquid Chromatographic Determination of Lamivudine, Indinavir and Ketoconazole in Dosage Forms and Biological Fluids. Pharm Anal Acta 2015; 6: 327
- Alozie O, Prosser R, Huppler Hullsiek K, Duprez D, Rhame F, Henry WK et. al. Switching from Abacavir/Lamivudine to Tenofovir DF/Emtricitabine Reduces Biomarkers of Inflammation: A Randomized Proof of Concept Study. J AIDS Clin Res 2014; 5: 278
- Lazarus EM, Otwombe K, Fairlie L, Untiedt S, Violari A, Fatima L et. al. Lamivudine Monotherapy as a Holding Strategy in HIV-Infected Children in South Africa. J AIDS Clin Res 2013; 4: 246
- Ali MM, Hasan F, Ahmad S, Al-Mufti S, Asker H, Farhan S, et. al. Mutation Patterns at Codons Rt204 And Rt180 of the HBV Polymerase Gene Associated with Lamivudine Resistance in Treated and Untreated Chronic HBV Patients in Kuwait: A Case Series. J Clin Case Rep 2013; 3:276
- Cuzin L, Allavena C, Finkielsztejn L, Melliez H, Pugliese P, Martin IP, et. al. Tolerance and Durability of Abacavir/Lamivudine (Abc/3tc) Containing Regimens: Results from a large French Prospective Cohort. J AIDS Clin Res 2012; S1: 019
- Dong BJ, Ward DJ, Chamberlain LA, Reddy YS, Ebrahimi R, Flaherty JF, et. al. Safety and Effectiveness of Tenofovir/Emtricitabine or Lamivudine Plus Ritonavir Boosted Atazanavir in Treatment Experienced HIV Infected Adults at Two Urban Private Medical Practices. J Antivir Antiretrovir 2012; 4: 001
- Feleder EC, Yerino GA, Halabe EK, Carla S, Soledad G, Zini Elvira. Single-Dose Bioequivalence of a New Fixed-Dose Combination Tablet Containing Tenofovir Disoproxil Fumarate and Lamivudine. 2011; 3: 236-243
- Amin J, De Lazzari E, Emery S, Martin A, Martinez E, Carr A,et. al. Simplification with Fixed-Dose Tenofovir-Emtricitabine or Abacavir-Lamivudine in Treatment Experienced, Virologically Suppressed Adults with Hiv Infection: Combined Analysis of Two Randomised, Non-Inferiority Trials Bicombo and Steal. J AIDS Clin Res 2010; 1:103
- P styletextalign. Bio Responsive In Situ Gel of Clindamycin for Vaginal Application. Journal of Pharmacy and Pharmaceutical Sciences 2013; 3:152
- Pawar Harshal A, Jadhav Surekha M, Jadhav Pravin T,Rachel G. Development and Evaluation of Mucoadhesive Patch Using a Natural Polysaccharide Isolated from Cordia dichotoma Fruit. J Mol Pharm Org Process Res 2014; 2:120
- Kumar GP, Geethika R, Anusha T, Jaweria S, Prathyusha G. The Potential of Statins for Buccal Delivery. J Mol Pharm Org Process Res 2014; 2: 111
- Suryakanta Swain. Mucoadhesive Micro and Nanoparticles for Oral Controlled Drug Delivery System for Prolongation of Gastric Residence and Its Application. Pharmaceut Reg Affairs 2012, 1:e115
- Nanjwade BK, Parikh KA, Deshmukh RV, Nanjwade VK, Gaikwad KR, Thakare SA, et. al. Development and Evaluation of Intranasal Mucoadhesiv Microspheres of Neostigmine Bromide. Pharm Anal Acta 2011; 2:118
- Nagai N, Yoshioka C, Tanabe W, Tanino T, Ito Y, Okamoto N, et. al. Effects of Ophthalmic Formulations Containing Cilostazol Nanoparticles on Retinal Vasoconstriction in Rats Injected with Endothelin-1. Pharm Anal Acta 2015; 6: 351
- Jacqueline BP, Lisa ST. Developing and Using a Case Formulation to Guide Cognitive-Behavior Therapy. J Psychol Psychother 2014; 5:179
- Gual A, Pau-Charles I, Abeck D. Topical Corticosteroids in Dermatology: From Chemical Development to Galenic Innovation and Therapeutic Trends. J Clin Exp Dermato 2015;6:269
- Ghaemi-Jandabi M, Abdollahi H, Azizi H, Sadeghizadeh M, Semnanian S. Dendrosomal Curcumin Nanoformulation Attenuates Naloxone Precipitated Morphine Withdrawal Signs in Rats. J Addict Res Ther 2015; 6:211
- Ojiako OA, Chikezie PC, Ogbuji AC. (2015) Glycemic Indices/Renal and Hepatic Antioxidant Status of Hyperglycemic Rats Treated with Single and Combinatorial Herbal Formulations. J Diabetes Metab 2015; 6:508
- Bhambere D, Shirivastava B, Sharma P, Gide P. Effect of Polymer and Formulation Variables on Properties of Self- Assembled Polymeric Micellar Nanoparticles. J Nanomedine Biotherapeutic Discov 2014; 4:129
- M Luiacutesa S Silva. Comprehensive Analysis of Phytopharmaceutical Formulations–An Emphasis on Two-Dimensional Liquid Chromatography. J Chromatogr Sep Tech 2015; 6: 262
- Sheikh S, Ahmad A, Ali SM, Ahmad MU, Paithankar M, Saptarshi D, et al. A New Topical Formulation of Minoxidil and Finasteride Improves Hair Growth in Men with Androgenetic Alopecia. J Clin Exp Dermatol Res 2015; 6: 253
- Fentie M, Belete A, Mariam TG. Formulation of Sustained Release Floating Microspheres of Furosemide from Ethylcellulose and Hydroxypropyl Methylcellulose Polymer Blends. J Nanomed Nanotechnol 2015; 5:262
- Sujana K, Hamuthal MZV, Murthy VSN, Shravani N. A Novel Validated Analytical Method Development for the Binary Mixture of Mebeverine and Chlordiazepoxide in Pharmaceutical Formulation and its Application to Stress Studies. Pharm Anal Acta 2015; 6: 324
- Nandini S, Nandini KE, Krishna Sundari S. Food and Agriculture Residue (FAR): A Potential Substrate for Tannase and Gallic Acid Production using Competent Microbes. J Bioprocess Biotech 2015; 5:193
- Singh A, Tandon S, Sand NK. Active Ingredient Estimation of Clopyralid Formulation by Reversed Phase HPLC. J Chromatogr Sep Tech 2015; 6: 257
- Sultana N, Saeed Arayne M, Shah SN. Development and Validation for the Simultaneous Quantification of Prazosin, Amlodipine, Diltiazem and Verapamil in API, Dosage Formulation and Human Serum by RP-HPLC: Application to in vitro Interaction Studies. Med chem 2014; 4: 770
- Murthy TGK, Geethanjali J. Development of a Validated RP-HPLC Method for Simultaneous Estimation of Metformin Hydrochloride and Rosuvastatin Calcium in Bulk and In-House Formulation. J Chromatogr Sep Tech 2014; 5: 252
- Datar P. Development of Opthalmic Formulation for Dry Eye Syndrome. Adv Pharmacoepidemiol Drug Saf 2014; 3:166
- Victoria F Samanidou. Various Aspects in the Impurity Profiling of Pharmaceutical Formulations. Pharm Anal Acta 2014; 5: e163
- Heidi I.G. AboElnaga. Photosynthetic Efficiency Promotion of Sugar Beet by Formulation of Trichoderma and Control of Some Sugar Beet Disease Seedling. Agrotechnol 2014; 3: 127
- Hooi Ling Ho. Effects of Medium Formulation and Culture Conditions on Microbial Xylanase Production Using Agricultural Extracts in Submerged Fermentation (SmF) and Solid State Fermentation (SsF): A Review . J Biodivers Biopros Dev 2014; 1:130
- Damodar R, Movva B, Mallikarjun PN, Pasumarthy C, Kona N. Formulation and Evaluation of Fast Dissolving Tablets of Diclofenac Sodium by Novel Hole Technology. J Mol Pharm Org Process Res 2014; 2:116
- Hoang Van Luong. Promotion of the Stability of Lycopene by Liposomal formulations for Anti-aging Therapy. Aging Sci 2014; 2: e113
- Rakesh Das, Tapan Kumar Pal. Assessment of Endogenous Biochemical Composites Emphasizing Drug Interaction of Cardiovascular Combined Dosage Formulation in Marketed Product. J Pharmacovigil 2014; 2:150
- Gnana Raja M. A Concise Study of Organic Volatile Impurities in Ten Different Marketed Formulations by [GC/HS-FID/MS] Gas Chromatography Technique. J Anal Bioanal Tech 2014; 5:202
- Nasar KM, Arifuddin, Tabassum K, Yasmin. A Comparative Clinical Trial on Leech Therapy and Unani Herbal Formulation on Atopic Eczema. J Clin Exp Dermatol Res 2014; 5: 232
- Rita M Hadley, Alan P Dine. Where is the Evidence? A Critical Review of Bias in the Reporting of Clinical Data for Exparel: A Liposomal Bupivacaine Formulation. J Clinic Res Bioeth 2014, 5:189
- Noriaki Nagai, Yoshimasa Ito. A New Preparation Method for Ophthalmic Drug Nanoparticles. Pharm Anal Acta 2014; 5: 305
- Nilambari S Patil, Jyoti P Jadhav. Single Cell Protein Production Using Penicillium ochrochloron Chitinase and Its Evaluation in Fish Meal Formulations. J Microbial Biochem Technol 2014; S4-005
- Hafez HM. Quantitative Determination of Amlodipine Besylate, Losartan Potassium, Valsartan and Atorvastatin Calcium by HPLC in their Pharmaceutical Formulations. J Chromat Separation Techniq 2014; 5: 226
- Seetharaman R, Lakshmi KS. Development and Validation of a Reverse Phase Ultra Performance Liquid Chromatographic Method for Simultaneous Estimation of Nebivolol and Valsartan in Pharmaceutical Capsule Formulation. J Chromat Separation Techniq 2014; 5: 229
- Hafez HM, Elshanawane AA, Abdelaziz LM, Kamal MM. Quantitative Determination of Amlodipine Besylate, Losartan Potassium, Valsartan and Atorvastatin Calcium by HPLC in their Pharmaceutical Formulations. Pharm Anal Acta 2014; 5: 300
- Scodeller P. Hyaluronidase and other Extracellular Matrix Degrading Enzymes for Cancer Therapy: New Uses and Nano-Formulations. J Carcinog Mutagen 2014; 5: 178
- Leonel ED, Sergio GFC. Cohesive Discontinuities Growth Analysis using a Nonlinear Boundary Element Formulation. J Appl Computat Math 2014; 3: 172
- David J Mastropietro, Hossein Omidian. Drug Tampering and Abuse Deterrence. J Develop Drugs 2014; 3:119
- Rother M, Vester J, Bolten WW, Kneer W, Conaghan PG. Meta-Analysis of Randomized Clinical Trials Investigating the Effect of TDT 064, a Gel-Based Formulation Containing Ultra-Deformable Phospholipid Vesicles, in Patients with Knee Osteoarthritis. Rheumatology (Sunnyvale) 2014; 4: 138
- Larramendy ML, Nikoloff ML, de Arcaute CR, Soloneski S. Genotoxicity and Cytotoxicity Exerted by Pesticides in Different Biotic Matrices-An Overview of More Than a Decade of Experimental Evaluation. J Environ Anal Toxicol 2014; 4:225
- Farid Menaa. Emulsions Systems for Skin Care: From Macro to Nano-Formulations. J Pharma Care Health Sys 2014; 1: e104
- Naveed S, Dilshad H, Jaweed L. Comparative Study of Four Different Brands of Ranitidine Available in Karachi. Mod Chem Appl 2014; 2: 125
- Urszula Domaska, Mohammed Halayqa. Promazine Hydrochloride/PLGA Biodegradable Nanoparticles Formulation and Release. J Phys Chem Biophys 2014; 4: 143
- Enriquez GG, Orawiec BA, Rizvi SAA, Do DP. Formulation Development and In vitro Evaluation of Oral Extended release Capsules Containing Biodegradable Microspheres. J Nanomed Nanotechnol 2014; 5:208
- Pan FT, Wu WZ, Zhi QG, Liang QX. Challenging Approach with Nanoformulation and Photodynamic Therapy in Dermatology. J Clin Exp Dermatol Res 2014; 5: 221
- Nehad A Abdallah. Validated Stability-indicating HPLC and Thin Layer Densitometric Methods for the Determination of Pazufloxacin: Application to Pharmaceutical Formulation and Degradation Kinetics. J Chromat Separation Techniq 2014; 5: 218
- Arasteh K, Drulak M, Guo J, Livrozet JM, Orkin C, Quinson AM, et al. TRANxITION 144 Week Results: Switching Virologically Stable HIV Patients from Immediate-release Nevirapine (NVP IR) to Extended-Release NVP (XR). J AIDS Clin Res 2014; 5: 292
- Fatima Altayib Alasha Abdalla, Abdalla Ahmed Elbashir. Development and Validation of Spectrophotometric Methods for the Determination of Mesalazine in Pharmaceutical Formulation. Med chem 2014; 4: 361
- Suresh Babu VV, Sudhakar V, Murthy TEGK. Validated HPLC Method for Determining Related Substances in Compatibility Studies and Novel Extended Release Formulation for Ranolazine. J Chromat Separation Techniq 2014; 5: 209
- Saeed Arayne M, Shahnaz H, Ali A, Sultana N. Monitoring of Pregabalin in Pharmaceutical Formulations and Human Serum Using UV and RP-HPLC Techniques: Application to Dissolution Test Method. Pharm Anal Acta 2014; 5: 28
- Sultana N, Naveed S, Saeed Arayne M. An Ultra-Sensitive and Selective LC-UV Method for the Simultaneous Determination of Metformin, Pioglitazone, Glibenclamide and Glimepride in API, Pharmaceutical Formulations and Human Serum. J Anal Bioanal Tech 2014, 5:176
- Naveed S. An Overview of Analytical Determination of Captopril in Active Pharmaceutical Ingredients (API) Formulation and Biological Fluids. J Bioequiv Availab 2013; 5: 264-266
- Gurupadayya BM, Disha NS. Stability Indicating HPLC Method for the Simultaneous Determination of Ceftriaxone and Vancomycin in Pharmaceutical Formulation. J Chromat Separation Techniq 2013; 4: 20
- Damle B, Tarabar S, Kuruganti U, Crownover P, LaBadie RR. Bioequivalence of Alprazolam Sublingual Tablet Formulation and Alprazolam Immediate Release Tablet in Healthy Volunteers. J Bioequiv Availab 2013; 5: 149-153
- Patelia EM, Rakesh Jayesh PT. Estimation Of Balsalazide By HPTLC-Densitometry Method In Pharmaceutical Formulation . J Chromat Separation Techniq 2013; 4: 189
- Sheikh REl, Amin AS, Gouda AA, Youssef AG. Utility of Oxidation-Reduction Reaction for Determination of Gemifloxacin Mesylate and Moxifloxacin HCl in Pure Form and in Pharmaceutical Formulations using N-Bromosuccinimide. Pharmaceut Anal Acta 2013; 4: 240
- Sultana N, Naveed S, Arayne MS. Facile and Manifest Liquid Chromatographic Method for the Simultaneous Determination of Enalpril Maleate and NSAIDs in API and Pharmaceutical Formulations. Pharm Anal Acta 2013; S2:004
- Sichel L, Timoshok NA, Pidgorskyy VS, Spivak NY. Study of Interferonogenous Activity of the New Probiotic Formulation Del-Immune V®. J Prob Health 2013; 1:107
- Vieillard V, Ghorbel N, Deffaux C, Astier A, Yiou R,Paul M. Development and Validation of a Stability- Indicating High Pressure Liquid Chromatography Method for Determination of Prostaglandin E1 and its Degradation Products in sn Intracavernous Formulation. Pharmaceut Anal Acta 2013; 4: 230
- Maheswari PD, Rambhau D, Narasu ML. Micellar Solubilization in the Formulation Development of Poorly Soluble Naproxen. Pharmaceut Reg Affairs 2013; 2: 108
- Attia Shafie MA, Mohammed Fayek HH. Formulation and Evaluation of Betamethasone Sodium Phosphate Loaded Nanoparticles for Ophthalmic Delivery. J Clin Exp Ophthalmol 2013; 4: 273
- Sultana N, Saeed Arayne M, Nadir Ali S, Tabassum A . Simultaneous Liquid Chromatographic Determination of Two Co- Prescribed Anti-Cancer Drugs in Bulk Drug, Dosage Formulations and in Human Serum Using Multivariate Technique: Application to in vitro Drug Interaction. Pharmaceut Anal Acta 2013; 4: 215
- Bhatt KK, Patelia EM, Mori. Simultaneous Estimation of Pregabalin and Methylcobalamine in Pharmaceutical Formulation by RP-HPLC Method. J Anal Bioanal Tech 2012; 4:159
- Abdalla Ahmed Elbashir, Salwa Fwad Awad. A New Spectrophotometric Method for Determination of Penicillamine in Pharmaceutical Formulation Using 1, 2-naphthoquine-4-sulfonate (NQS). J Pharmacovigilance 2013; 1: 105
- Menon S, Kandari K, Mhatre M, Nair S. Bioequivalence and Pharmacokinetic Evaluation of Two Formulations of Armodafinil 250 mg Tablets in Healthy Indian Adult Male Subjects. J Bioequiv Availab 2013; 5: 095-098
- Bhople A, Deshpande S, Sheiakh S, Patange H, Chandewar A, Patil S. Formulation and Development of Ketorolac Tromethmaine Ophthalmic Solution. J Bioequiv Availab 2013; 5: 099-109
- Shah DA, Patelia EM, Mori A. Simultaneous Estimation of Pregabalin and Methylcobalamine in Pharmaceutical Formulation by HPTLC-Densitometry Method. J Chromat Separation Techniq 2012, 4: 169
- Mehta FA, Patelia EM, Bhoya PN. Simultaneous Estimation of Ambroxol Hydrochloride and Doxofylline in Pharmaceutical Formulation by HPTLC-Desitometric Method. J Chromat Separation Techniq 2012, 4: 168
- Ahir KB, Patelia EM, Shah A. Simultaneous Estimation of Metformin Hydrochloride and Repaglinide in Pharmaceutical Formulation by HPTLC-Densitometry Method. J Chromat Separation Techniq 2012, 4: 16
- Kiselev V, Klinskiy E, Lee S, Muyzhnek E, Semov A,Alakhov V. Polymer Based Nano-Formulation of Diindolylmethane with High Oral Bioavailability. J Nanomed Nanotechnol 2013, 4:162
- Wagh N, Gayakwad NJ, Christina AJM, Bhople A, Thakre A. A Bioequivalence Study of Two Finofibrate Tablet Formulations in Indian Healthy Subjects. J Bioequiv Availab 2013, 5: 016-02
- Zhao Q, Purohit V, Cai J, LaBadie RR, Chandra R. Relative Bioavailability of a Fixed-Combination Tablet Formulation of Azithromycin and Chloroquine in Healthy Adult Subjects. J Bioequiv Availab 2013, 5: 001-005
- Bassam Abdul Rasool Hassan. Overview on Pharmaceutical Formulation and Drug Design. Pharm Anal Acta 2012; 3:e140
- Lokesh PNV, Abdul Althaf S, Sailaja PB. Design, Development and Formulation of Orodispersible Tablets of a Model Drug Using Response Surface Methodology. Pharm Anal Acta 2012; 3:195
- Abdul Althaf S, Sailaja PB, Ashwin Kumar M. Formulation, Evaluation and Mathematical Modelling of Clopidogrel Bisulphate & Aspirin Immediate Release Bilayer Tablets. Pharm Anal Acta 2012; 3:194
- Ahir KB, Patelia EM, Mehta F. Simultaneous Estimation of Tramadol HCl, Paracetamol and Domperidone in Pharmaceutical Formulation by RP-HPLC Method. J Chromat Separation Techniq 2012, 3:152
- Sultana N, Arayne MS, Ali SN. An Ultra-Sensitive LC Method for Simultaneous Determination of Rosuvastatin, Alprazolam and Diclofenac Sodium in API, Pharmaceutical Formulations and Human Serum by Programming the Detector. J Anal Bioanal Techniques 2012, 3:154
- Behera S, Ghanty S, Ahmad F, Santra S, Banerjee S. UV-Visible Spectrophotometric Method Development and Validation of Assay of Paracetamol Tablet Formulation. J Anal Bioanal Techniques 2012, 3:151
- Madhavi N, Sudhakar B, Ravikanth PV, Mohon K, Ramana Murthy K. Formulation and Evaluation of Phenytoin Sodium Sustained Release Matrix Tablet. J Bioequiv Availab 2012, 4: 128-133
- Bartoli AN, De Gregori S, Molinaro M, Broglia M, Tinelli C, et al. Bioavailability of a New Oral Spray Melatonin Emulsion Compared with a Standard Oral Formulation in Healthy Volunteers. J Bioequiv Availab 2012, 4: 096-099
- Moghaddam AB, Mohammadi A, Mohammadi S. Electroanalysis of Mefenamic Acid in Pharmaceutical Formulation and Spiked Biological Fluids on Modified Carbon Nanotube Electrode. Pharm Anal Acta 2012; 3:165
- Ahir KB, Patelia EM, Mehta FA. Development of a Validated Stability- Indicating HPTLC Method for Nitazoxanide in Pharmaceutical Formulation. J Chromat Separation Techniq 2012, 3:138
- Ahir KB, Patelia EM, Mehta FA. Simultaneous Estimation of Tramadol Hcl, Paracetamol and Domperidone in Pharmaceutical Formulation by Thin-Layer Chromatographic Densitometric Method. J Chromat Separation Techniq 2012, 3:139
- Walker GM. Advances in Pharmaceutical Formulation and Processing. J Chem Eng Process Technol 2012, 3:e109
- Kumar P, Ghosh A, Chaudhary M. Stability Indicating Method Development for Simultaneous Estimation of Ezetimibe and Atorvastatin in Pharmaceutical Formulations by RP-HPLC. Pharm Anal Acta 2012, 3:164
- Mukesh P Ratnaparkhi. Formulation and Development of Taste Masked Orally Disintegrating Tablets of Perindopril Erbumine by Direct Compression Method. Pharm Anal Acta 2012, 3:162
- Bhaskar R, Bhaskar R, Sagar MK, Saini V, Bhat KM. UV-Spectrophotometric-Assisted Chemometric Methods for the Simultaneous Determination of Metformin Hydrochloride and Gliclazide in Pharmaceutical Formulations. Pharm Anal Acta 2011, 3:158
- Dalapathi B Gugulothu, Vandana B Patravale. A New Stability-Indicating HPLC Method for Simultaneous Determination of Curcumin and Celecoxib at Single Wavelength: an Application to Nanoparticulate Formulation. Pharm Anal Acta 2012, 3:157
- Safila Naveed, Najma Sultana, M.Saeed Arayne. HPLC-UV Method for the Determination of Enalapril in Bulk, Pharmaceutical Formulations and Serum. J Anal Bioanal Techniques 2012, 3:130
- Naveen Kumar Reddy G, Rajendra Prasad VVS, Maiti NJ, Nayak D, Prashant Kumar M. Development and Validation of a Stability Indicating UPLC Method for Determination of Moxifloxacin Hydrochloride in Pharmaceutical Formulations. Pharm Anal Acta 2011, 2:14
- Jain PS, Tatiya AU, Bagul SA, Surana SJ. Development and Validation of a Method for Densitometric Analysis of 6-Gingerol in Herbal extracts and Polyherbal Formulation. J Anal Bioanal Techniques 2011, 2:124
- Whitmire M, Ross R, Mwalimu J, Porter L, Whitsel M. A Global GLP Approach to Formulation Analysis Method Validation and Sample Analysis. Pharm Anal Acta 2011, S2:001.
- Abdelkawy M, Metwaly F, El Raghy N, Hegazy M, Fayek N. Simultaneous determination of Ambroxol Hydrochloride and Guaifenesin by HPLC, TLC-Spectrodensitometric and multivariate calibration methods in pure form and in Cough Cold Formulations. J Chromatograph Separat Techniq 2011, 2: 112
- Nanjwade BK, Derkar GK, Bechra HM, Nanjwade VK, Manvi FV. Design and Characterization of Nanocrystals of Lovastatin for Solubility and Dissolution Enhancement. J Nanomed Nanotechnol 2011, 2:107
- Devika GS, Sudhakar M, Venkateshwara Rao J. Simultaneous Determination of Eprosartan Mesylate and Hydrochlorthiazide in Pharmaceutical Dosage form by Reverse Phase High Performance Liquid Chromatography. Pharm Anal Acta 2011, 2:122
- Anis SM, Hosny MM, Abdellatef HE, El-Balkiny MN. Kinetic Spectrophotometric Determination of Betahistine Dihydrochloride and Etilefrine Hydrochloride in Pharmaceutical Formulation. Pharm Anal Acta 2011, 2:116
- Manassra A, Khamis M, el-Dakiky M, Abdel-Qader Z, Al-Rimawi F. Simultaneous HPLC Analysis of Betamethasone and Clotrimazole in Cream Formulations. Pharm Anal Acta 2010, 1:113
- Prabu SL, Srinivasan M, Thiagarajan S, Marina Q. Simultaneous Determination of Gatifloxacin and Ambroxol Hydrochloride in a Tablet Formulation by Liquid Chromatography. Pharm Anal Acta 2010, 1:110
- Subbaiah PR, Kumudhavalli MV, Saravanan C, Kumar M, Chandira RM. Method Development and Validation for estimation of Moxifloxacin HCl in tablet dosage form by RP-HPLC method. Pharm Anal Acta 2010, 1:109
- Al-Rimawi F. Validation of an HPLC-UV Method for the Determination of Amiodarone Impurities in Tablet Formulations. Pharm Anal Acta 2010, 1:105
- Santhoskumar AU, Palanivelu K, Sharma SK, Nayak SK. A New Synthesis of Nickel 12-Hydroxy Oleate Formulation to Improve Polyolefin’s Degradation. J Bioremed Biodegrad 2010, 1:108
- Saber AL, Amin AS. Utility of Ion-Pair and Charge Transfer Complexation for Spectrophotometric Determination of Domperidone and Doxycycline in Bulk and Pharmaceutical Formulations. J Anal Bioanal Techniques 2010, 1:113
- Dhaneshwar SR, Salunkhe JV, Bhusari VK. Validated HPTLC Method for Simultaneous Estimation of Metformin Hydrochloride, Atorvastatin and Glimepiride in Bulk Drug and Formulation. J Anal Bioanal Techniques 2010, 1:109
- Salvi VS, Sathe PA, Rege PV . Determination of Tinidazole and Ciprofloxacin Hydrochloride in Single Formulation Tablet using Differential Pulse Polarography. J Anal Bioanal Techniques 2010, 1:110.
- Komano Y, Yagi N, Nanki T. Joint-Targeting Drug Delivery System for Rheumatoid Arthritis: siRNA Encapsulated Liposome. Pharm Anal Acta 2015, 6: 352
- Agrawal P. Significance of Polymers in Drug Delivery System. J Pharmacovigil 2015, 3:e127
- Jigar N Shah, Hiral J Shah, Anastasia Groshev, Anjali A Hirani, Yashwant V Pathak et al. Nanoparticulate Transscleral Ocular Drug Delivery. J Biomol Res Ther 2015, 3: 116
- Malika V, Kohli K, Chaudhary H, Kumar V. Nano-Carrier for Accentuated Transdermal Drug Delivery. J Develop Drugs 2014, 3:121
- Gavasane AJ, Pawar HA. Synthetic Biodegradable Polymers Used in Controlled Drug Delivery System: An Overview. Clin Pharmacol Biopharm 2014, 3:12
- Silva HR, Luz GM, Satake CY, Correa BC, Sarmento VHV, Oliveira GH, et al. Surfactant-based Transdermal System for Fluconazole Skin Delivery. J Nanomed Nanotechnol 2014, 5:231
- Nagai N, Ito Y. A New Preparation Method for Ophthalmic Drug Nanoparticles. Pharm Anal Acta 2014, 5: 305
- Xiang Q, Morais PC. Remote Hyperthermia, Drug Delivery and Thermometry: The Multifunctional Platform Provided by Nanoparticles. J Nanomed Nanotechnol 2014, 5:209
- Singh M, Kumar M, Manikandan S, Chandrasekaran N, Mukherjee A, Kumaraguru AK. Drug Delivery System for Controlled Cancer Therapy Using Physico-Chemically Stabilized Bioconjugated Gold Nanoparticles Synthesized from Marine Macroalgae, Padina Gymnospora. J Nanomed Nanotechol 2014, S5-009
- Tyrrell J, Tarran R. Gaining the Upper Hand on Pulmonary Drug Delivery. J Pharmacovigilance 2014, 2:118
- Phan CM, Hui A, Subbaraman L, Jones L. Insights to Using Contact Lenses for Drug Delivery. Clin Exp Pharmacol 2013, 4: 145
- Gundogdu E, Baspinar Y, Koksal C, Ince I, Karasulu E. A Microemulsion for the Oral Drug Delivery of Pitavastatin. Pharmaceut Anal Acta 2013; 4: 209
- Rasool Hassan BA . Overview on Drug Delivery System. Pharm Anal Acta 2012; 3:e137
- Benyettou F, Milosevic I, Olsen JC, Motte L, Trabolsi A. Ultra-Small Superparamagnetic Iron Oxide Nanoparticles Made to Order. J Bioanal Biomed. 2012, S5: 006
- Amirthalingam T, Kalirajan J, Chockalingam A. Use of Silica-Gold Core Shell Structured Nanoparticles for Targeted Drug Delivery System. J Nanomed Nanotechnol 2011, 2:119
- Saboktakin MR, Tabatabaie RM, Maharramov A, Ramazanov MA. Synthesis and Characterization of Biodegradable Thiolated Chitosan Nanoparticles as Targeted Drug Delivery System. J Nanomed Nanotechnol 2011, S4-001
- Miruka CO, Matunda NC, Ejekwumadu NJ, Mokembo JN. Design of a Recombinant Hepatitis B Vaccine Based on Stably Binding HLA-I Peptides. J Biomol Res Ther 2015, 4: 120
- Salvana EMT, Salvana AD, Salata RA. HIV with Hepatitis B Co-Infection: Optimizing Treatment in Resource-Limited Settings. J AIDS Clin Res 2015, 5: 425
- Wong SY, Hann HW. Anti-Viral Therapy can Prevent Recurrent Hepatocellular Carcinoma Associated with Hepatitis B: Recent Development. J Antivir Antiretrovir 2015, 7: 116
- Higgs G, Lin Z, Cento V, Svicher V, Hattangadi S, Zhang J. Using Bayesian Models to Locate Mutations for HBV Drug Resistance. J Hematol Thrombo Dis 2014, 2:166
- Yaron Z, Alex, Yehoda S. Infantile Bullous Pemphigoid Following Hepatitis B Vaccinations. Immunome Res 2014, 10:078
- Lv Y, Lau WY, Han X, Gong X, Ma Q, et al. Grading of Peripheral Cytopenias due to Splenomegaly and Hepatitis B Cirrhotic Portal Hypertension. J Hypertens 2013, 3: 182
- Mudoi P, Bharali P, Konwar BK. Study on the Effect of pH, Temperature and Aeration on the Cellular Growth and Xanthan Production by Xanthomonas campestris Using Waste Residual Molasses. J Bioprocess Biotech 3:135
- Babu B, Meyyanathan SN, Gowramma B, Muralidharan S, Elango K, et al. Pharmacokinetic Evaluation of Newly Developed Oral Immediate Release and Sustained Release Dosage Forms of Losartan Potassium. J Bioequiv Availab 4:121-127
- NHabibzadeh, Bolhassani A, Vahabpour R, Sadat SM. How can Improve DNA Vaccine Modalities as a Therapeutic Approach against HIV Infections? J AIDS Clin Res 6: 440
- Buczynski AK, Leão ATT, de Souza IPR. Evaluation of Quality of Life in HIV-Infected Children and Children with Cancer. Dentistry 5:276
- Ajao KO, Osho PO, Koledoye V, Fagbemi SO, Oluwatoyosi DO. Factors Influencing Condom Use among People Living with HIV/ AIDS Attending Clinics at State Specialist Hospital, Akure, Ondo State, Nigeria. Gynecol Obstet (Sunnyvale) 4:254
- Park LS, Tate JP, Rodriguez-Barradas MC, Rimland D, Goetz MB, et al. Cancer Incidence in HIV-Infected Versus Uninfected Veterans: Comparison of Cancer Registry and ICD-9 Code Diagnoses. J AIDS Clin Res. 2014, 5:318
- Nemutandani MS, Adedoja D, Nemutandani V. Aids Pandemic: Traditional Practices Increasing Risk of HIV Infections in South Africa. J Clin Res Bioeth 5:173
- Jeevani T, Aliya S. HIV Infections- Acquired Immuno Deficiency Syndrome Malignancies. J AIDS Clinic Res 2:131
- Nanjwade BK, Parikh KA, Deshmukh RV, Nanjwade VK, Gaikwad KR, et al. Development and Evaluation of Intranasal Mucoadhesiv Microspheres of Neostigmine Bromide. Pharm Anal Acta 2:118
- Silva MLS. Comprehensive Analysis of Phytopharmaceutical Formulations – An Emphasis on Two-Dimensional Liquid Chromatography. J Chromatogr Sep Tech. 2015, 6:262.
- Samanidou VF. Various Aspects in the Impurity Profiling of Pharmaceutical Formulations. Pharm Anal Acta. 2014, 5:e163.
- Mukthinuthalapati MA, Bukkapatnam V, Bandaru SPK, Grandhi NS. Simultaneous Determination of Rosuvastatin and Ezetimibe in pharmaceutical formulations by Stability Indicating Liquid Chromatographic Method. J Bioequiv Availab. 2014; 6:174-180
- Ghoneim M, Saber AL, El-Desoky H. Utility Spectrophotometric and Chromatographic Methods for Determination of Antidepressant Drug Sulpiride in Pharmaceutical Formulations and Plasma. J Anal Bioanal Tech. 2014, 5:183.
- Saeed Arayne M, Shahnaz H, Ali A, Sultana N. Monitoring of Pregabalin in Pharmaceutical Formulations and Human Serum Using UV and RPHPLC Techniques: Application to Dissolution Test Method. Pharm Anal Acta. 2014, 5:287.
- Kumar P, Ghosh A, Chaudhary M. Stability Indicating Method Development for Simultaneous Estimation of Ezetimibe and Atorvastatin in Pharmaceutical Formulations by RP-HPLC. Pharmaceut Anal Acta. 2012,3:164.
- Sultana N, Arayne MS, Ali SN. An Ultra-Sensitive LC Method for Simultaneous Determination of Rosuvastatin, Alprazolam and Diclofenac Sodium in API, Pharmaceutical Formulations and Human Serum by Programming the Detector. J Anal Bioanal Tech. 2012, 3:154
- Sultana N, Naveed S, Arayne MS. Facile and Manifest Liquid Chromatographic Method for the Simultaneous Determination of Enalpril Maleate and NSAIDs in API and Pharmaceutical Formulations. Pharm Anal Acta. 2013, S2:004
- Sheikh REl, Amin AS, Gouda AA, Youssef AG. Utility of Oxidation- Reduction Reaction for Determination of Gemifloxacin Mesylate and Moxifloxacin HCl in Pure Form and in Pharmaceutical Formulations using N-Bromosuccinimide. Pharm Anal Acta. 2013, 4:240
- Mastropietro DJ, Nimroozi R, Omidian H. Rheology in Pharmaceutical Formulations-A Perspective. J Develop Drugs. 2013, 2:108
- Sultana N, Naveed S, Saeed Arayne M. An Ultra-Sensitive and Selective LC-UV Method for the Simultaneous Determination of Metformin, Pioglitazone, Glibenclamide and Glimepride in API, Pharmaceutical Formulations and Human Serum. J Anal Bioanal Tech. 2013, 5: 176
- Bhaskar R, Bhaskar R, Sagar MK, Saini V, Bhat KM. UVSpectrophotometric- Assisted Chemometric Methods for the Simultaneous Determination of Metformin Hydrochloride and Gliclazide in Pharmaceutical Formulations. Pharmaceut Anal Acta. 2012, 3:158.
- Naveed S, Sultana N, Arayne MS.HPLC-UV Method for the Determination of Enalapril in Bulk, Pharmaceutical Formulations and Serum. J Anal Bioanal Tech. 2012, 3:130.
- Naveen Kumar Reddy G, Rajendra Prasad VVS, Maiti NJ, Nayak D, Prashant Kumar M. Development and Validation of a Stability Indicating UPLC Method for Determination of Moxifloxacin Hydrochloride in Pharmaceutical Formulations. Pharm Anal Acta. 2011, 2:142
- Devika GS, Sudhakar M, Venkateshwara Rao J. Simultaneous Determination of Eprosartan Mesylate and Hydrochlorthiazide in Pharmaceutical Dosage form by Reverse Phase High Performance Liquid Chromatography. Pharm Anal Acta. 2011, 2:122.
- Saber AL, Amin AS. Utility of Ion-Pair and Charge Transfer Complexation for Spectrophotometric Determination of Domperidone and Doxycycline in Bulk and Pharmaceutical Formulations. J Anal Bioanal Tech. 2010, 1:113.
- Govindarajan. Cassia roxburghii seed galactomannan: Potential binding agent for pharmaceutical formulations. 2014, 4th International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Michael Morgen. Amorphous drug-polymer nanoparticles: An enabling drug delivery platform. 2014, 4th International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Cassia roxburghii seed galactomannan: Potential binding agent for pharmaceutical formulations. 2014, 4th International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Amorphous drug-polymer nanoparticles: An enabling drug delivery platform. 2014, 4th International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Jason Vaughn. Stepwise preformulation to ensure successful product development of oral solid and semisolid dosage forms. 2014, 4th International Conference and Exhibition on Page 45 Pharmaceutics & Novel Drug Delivery Systems
- Yanwei Zhong, Shishu Zhu, Hongfei Zhang, Dongping Xu, Yi Dong, Dawei Chen et al. Virologic and clinical characteristics of HBV genotypes/ subgenotypes in chinese pediatric patients with chronic Hepatitis B. VIROLOGY. 2011, International Conference and Exhibition on Virology
- Nguemaim N, Mbuagbaw J, Teto G, Nkoa T, Same-Ekobo A, Asonganyi. F Morphological changes in HIV-1 infected patients on antiretroviral therapy without protease inhibitors in Cameroon: A prospective cohort study. 2013, 2nd International Conference on Clinical & Cellular Immunology
- Princy Louis Palatty, Sana Aboobaker, Manasa S, Scandashree. Comparative pharmacokinetics and compliance issues to optimize art- The Indian scenario. 2013, 2nd International Summit on GMP, GCP & Quality Control
- Kennedy D Mwambete. Neglected tropical diseases causes of health concern for HIV/AIDS patients. 2014, 3rd International Conference on Clinical Microbiology & Microbial Genomics
- Sandeep B. Study of self-reported Adverse Drug Reactions and factors influencing it among HIV/AIDS patients on antiretroviral therapy. 2014, 3rd International Conference and Exhibition on Pharmacovigilance & Clinical Trials
- Leela Rani Gannavarapu, Haritha Singh Khastri. Design and optimization of sustained release matrix tablets of Lamivudine. 2013, 3rd International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Husham. Trial of lamivudine in hepatitis-B surface antigen carriers with persistence hepatitis-B core IgM antibody. 2012,World Congress on Gastroenterology & Urology
- Divya Sree, Thadkapally Srinivas, Aramalla Eashwar Rao. Method development and validation of the assay and dissolution of a fixed dose combination of tenofovir and efavirenz tablet. 2012, 2nd International Conference and Exhibition on Pharmaceutical Regulatory Affairs
|