ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
D Sarvamangala1, Kantipriya Kondala2, U S N Murthy3, B Narasinga Rao4, N Sivakumar5
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
The present day drug market is almost inundated with a plethora of eye drops and eye ointments to answer prescriptions for curing ocular diseases. A path breaking and innovative thinking is towards employing silver nanoparticles (AgNP’S) effectively and advantageously for sustained drug delivery system, in the prophylactic as well as curative treatment of ophthalmic indispositions. In this present work, silver nanoparticles obtained through leaf extract of Alternanthera sessilis (A.sessilis) presented a size of 3 to 50 nm and were round in shape. Particles thus obtained were subjected to various series of tests viz. UV-Visible Absorption Spectroscopy (UV-Vis), X-Ray Diffraction (XRD), Scanning Electron Microscope (SEM), Energy Dispersive Spectroscope (EDS), Particle Size Analysis (PSA) and Transmission Electron Microscope (TEM) analysis. It is observed that the synchronous action of AgNP’S assures prevention as well as eradication of ocular pathogens bereft of any side effect and identified the synergetic activity of silver nanoparticles against ocular pathogens. Besides, drugs developed by using silver nanoparticles, have specific biological targets such as replicative, synthetic or metabolic enzymes which are unique to disease causing microbes and can be safely introduced into living tissues. Plentiful evidence is available to assert that plenitude of protection against ocular pathogens is provided by AgNP’S, adequately safeguarding intraocular efficacy. The rationale behind the application of silver nanoparticles (AgNP’S) against eye pathogens is – it is cost effective and eco-friendly. Research work on silver nanoparticles against eye pathogens will provide an answer to prevent devastating sight – threatening pathogenic complications in the eye.
Keywords |
| Alternanthera sessilis, Ocular diseases, Silver nanoparticles, synergetic activity, Transmission Electron Microscope. |
I. INTRODUCTION |
| Various anatomical and physiological structures that constitute the structure of eye are very special with independent functions. For example, the cornea and the crystalline lens have no blood supply but play a key role in functioning of the eye[1]. The drug delivery into the eye poses many problems to the pharmaceutical scientists due to the complex structure of the organ. Entry of drug molecules at the target area for action is limited by the unique structure of eye[2]. Recent advancement in drug delivery approaches and material sciences have enabled scientists to invent new therapies for treatment of eye diseases. In the conventional medical system the ocular diseases were mostly treated by systemic administration or intraocular or periocular injections. Traditionally eye treatment involved application of solutions, suspensions, gels, ointments and inserts [3] which suffered from poor corneal permeability, nasolacrimal drainage and systemic absorption resulting in blurred vision [4]. The new approach based on Nano carriers is now extensively investigated as an attractive and desirable system of treatment of eye since particulated delivery systems such as nanoparticles improve the pharmacokinetic and pharmacodynamic properties of ocular drug particles [5]. The efficiency of drug delivery increases by novel systems such as nanoparticles which show more benefits over conventional systems. These new systems improve the release profile of the drug and reduce its toxicity. |
| It is realized, with the rapid progress of the biosciences, the new possibilities to meet the needs of the posterior segment treatments are fairly available. The first nanoparticle delivery system studied was Piloplex, consisting of Pilocarpine ionically bound to Poly (methyl) methacrylate acrylic acid copolymer nanoparticles [6-7]. In the present study, we have taken silver nitrate to produce Ag nanoparticles in leaf extract medium because silver nitrate is a prophylaxis for ophthalmic diseases and at present silver has been recognized for its inhibitory action on microbes in medical and industrial process [8-9]. With an advanced technology, investigations have been carried out in recent times based on the use of nanoparticles with an aim to improve Effective front of the eye drug delivery system [10-11]since this system provides prolonged residence time at the ocular surface and reducing the effect of natural eye clearance systems. In several excellent texts, the use of nanoparticles and nanocarriers of ocular drug delivery have been reviewed [12]. The nanoparticles used are synthesized through biological methods to avoid side effects and other complications. There are no reports so far on the use of silver nanoparticles synthesized by A.sessilis leaf extract with addition of ocular drugs and here we report a novel approach that has high potential in drug delivery. |
| The weed plant named “Alternanthera sessilis” or “sessile joy weed” commonly called as “ponnaganti” which belongs to “Amarantheacea” family used in the present study is referred to as one of the greatest multipurpose miracle herb of oriental medicine capable of curing burning sensation in stomach, diarrhoea, skin diseases, dyspepsia, haemorrhoids and treating several liver and spleen diseases. It has many pharmacological therapeutic effects. Nanoparticles prepared were characterized by UV- Vis Absorption Spectroscope, XRD, EDS, SEM, PSA and TEM.The silver nanoparticles prepared in this study were tagged to ophthalmic antibiotics and these were investigated against different multi drug resistant ophthalmic eye pathogens. |
II. LITERATURE SURVEY |
| The use of environmentally benign materials like plant leaf extract bacteria and fungi for the synthesis of silver nanoparticles offers numerous benefits of eco-friendliness (V. Parashar et al., 2009; N. Saifuddin et al., 2009; C. Bhainsa et al., 2006). Silver is highly toxic to most of the microbial cells (S. Silver., 2003). Nanoparticles of well- defined size, ranging from a few to 200nm or more and distinct morphology are deposited within the periplasmic space of the bacteria. Cell growth and metal incubation conditions may be the reasons for the formation of different particle sizes (Silver S., 1996). In 1884 German obstetrician C.S.F. crede introduced 1% silver nitrate as an eye solution for prevention of Gonococcal ophthalmic neonatorum, which is perhaps the first scientifically documented medical use of silver (A.D.Russell et al., 1994). It is well known that silver nanoparticles exhibit brown colour- in aqueous solution due to excitation of surface Plasmon vibrations is silver – nanoparticles (Afreen et al., 2011). It is generally recognized that UV VIs spectroscopy could be used to examine size and shape controlled nanoparticles in aqueous suspensions (S.S. Shankar et al., 2004). The synthesis of Nano silver by the plant extract was further characterized by EDS analysis gave additional evidence for the reduction of silver nanoparticles to elemental silver (Xie J et al., 2007). |
| The first nanoparticulate delivery system studied was Piloplex, consisting of Pilocarpine ionically bound to poly methacrylate-acrylic aciol copolymer nanoparticles (Kreuter J et al., 1997). The maximum size limit for micro particles for ophthalmic administration is about 5-10nm above which a scratching feeling in the eye can result upon ocular instillation (Patel Vishal et al., 2011) nanoparticles are carriers for ophthalmic application (Kreutar J., 1978) An optimal corneal penetration of the encapsulated drug was reported in presence of bioadhesive polymer chitosan (VOD et al., 1997). |
III. MATERIALS AND METHODS |
| 1. Experimental: Silver nitrate wasobtained from Finar Production Company. All the glassware used in the present work was sterilized inhot air oven before use. |
| 2. Plant material:A.sessilis is a profusely branched perennial herb that grows spreading over ground, simple leaves with small and white flowers in axillary clusters and distributed throughout India in moist places, growing wild and often cultivated. Plant material contains 5% of iron i.e., 16.7mg/100g and a good amount of alpha – tocopherols & beta – tocopherols. Fresh leaves of A.sessilis were collected from Srikakulam & Visakhapatnam areas, Andhra Pradesh, India. The plant specimen was identified and certified by Dept. of Botany, Andhra University, Visakhapatnam. |
| 3. Preparation of leaf extract: The fresh leaves weighing 150gms of A.sessilis were washed several times with distilled water to remove dust, cut into fine pieces and were crushed into300 ml sterile distilled water and filtered through Whatman No.1 filter paper. The filtration process was repeated and used for synthesis of silver nanoparticles. |
| 4. Synthesis of nanoparticles:1mM aqueous solution of silver nitrate was prepared and used for the synthesis of silver nanoparticles. 10mL of leaf extract was added into90mLof 1mMsilver nitrate. The primary detection of synthesized silver nanoparticles was carried out in the reaction mixture by observing the color change from greenish to dark brown and optical density (O.D) at different time intervals were takenfor 6 hours, using a UV – Visible Spectroscopy. Then the solution is stored in dark at room temperature for 24 hours for the complete settlement of nanoparticles. After 24 hours, the reaction mixture was centrifuged at 10,000 rpm for 10 minutes. The suspension concentrated by repeated centrifugation. The supernatant was replaced by 10ml of distilled water each time and suspension stored for antibacterial assays and for the optical measurements. |
| 5. Test organism: The eye pathogens Staphylococcus aureus and Pseudomonas aeruginosa were obtained from Department of Microbiology, Rajiv Gandhi Institute of Medical Sciences (RIMS), Srikakulam, A.P, India and used for the invitro antibacterial sensitivity tests. |
Analysis of silver nanoparticles: |
| i. UV – Visible Spectra Analysis: The bioreduction of reaction mixture of pure silver ions was observed by measuring theUV- Vis Spectrum at different time intervals taking 1mL of the sample, using 1mL of distilled water as blank. UV – VIS Spectrometer UV – 2450 (Shimadzu, Japan) was used. |
| ii. XRD Analysis: The suspension which is stored for optical measurements was purified by repeated centrifugation at 10,000 rpm for 10 minutes and freeze dried. The freeze-dried silver nanoparticles were analyzed by using Xpert PRO, X – ray diffractometer (PANalytical BV) to determine the characterization of the nanoparticles by operation at a voltage of 40kv and the intensity of the diffracted x rays is measured as a function of the diffraction angle 2Ɵ. The crystalline domain size was calculated from the width of the XRD peaks, using the Scherer formula. |
| D = 0.94λ/βCOSƟ |
| Where D is the average crystalline domain size perpendicular to the reflecting planes, λ is the x-ray wavelength, β is the Full Width at Half Maximum (FWHM), and Ɵ is the diffraction angle. |
| iii. SEM Analysis: The morphological characterizations of the samples were done using JEOL (JSM – 6610LV) SEM machine. Thin films of the sample were prepared on carbon coated copper grid by just dropping a very small amount of the sample on the grid, extra solution was removed using a blotting paper and then the film on the SEM grid were allowed to dry by putting it under a mercury lamp for 5 minutes. In the analysis an electron beam is focused intoaffine probe and subsequently rasterscanned over a small rectangular area. As the beam interacts with the sample it creates various signals all of which can be appropriately detected. |
| iv. EDS Analysis: Energy Dispersive Spectroscope Analysis determined the presence of elemental silver. In order to carry out EDS Analysis, thin films of the sample were prepared on a carbon coated copper grid by just dropping a very small amount of the sample on the grid and performed on JEOL JSM – 6610LV SEM instrument equipped with a thermo EDS attachment. |
| v. TEM Analysis: Transmission Electron Microscopic (TEM) Analysis was performed with JEOL 1200 EX instrument operating at 120kv voltage. Thin film of the sample were prepared on a carbon coated grid by dropping a very small amount of the sample on the grid, extra solution was removed using a blotting paper. Later on, film on the TEM grid was allowed to dry by placing it under a mercury lamp for 5 minutes for the characterization of size and shape of synthesized silver nanoparticles. |
| vi. PS Analysis: The particle size ranges of the nanoparticles were determined by using Particle Size Analyzer (nano partica SZ – 100, horiba, Japan). Particle sizes were arrived based on measuring the time dependent fluctuation of scattering of laser light by the nanoparticles undergoing Brownian motion. |
| vii. Antibacterial Assays: The antibacterial assays by agar diffusion method was done against human ophthalmic pathogenic bacteria like Staphylococcus aureus, Pseudomonas aeruginosa lawn cultures on Mueller Hinton (MH) agar plates using turbidity standards and fresh overnight cultures were used for inoculating silver nanoparticles. 10μL of sample was inoculated on MH agar plates. The zone of inhibition around area of silver nanoparticle was measured after 18 – 24h with Antimicrobial Sensitivity Measuring scale and the absence of growth on the plates around the silver nanoparticle area confirmed antibacterial activity. |
| Here we report the effect of silver nanoparticles extracted from A.sessilis, when they are attached to ocular antibiotics like Gatifloxacin and Tobramycin in the ratio of 1:0.5, 1:1 and 1:2 againstS.aureus and P.aereginosa. |
IV. RESULTS AND DISCUSSION |
| The A.sessilis plant leaf extract was used for the synthesis of silver nanoparticles. The reaction started with in first hour of the incubation with silver nitrate (1mM). This was confirmed by the appearance of brown colour in the reaction mixture. |
| 1. Visual Inspection: It is well known that silver nanoparticles exhibit brown color in aqueous solution due to excitation of Surface Plasmon vibrations. It was found that aqueous silver ions when exposed to A.sessilis leaf extract were reduced in solution, there by leading to the formation of silver hydrosol. The leaf extract was green in color before the addition of silver ions and this changed to dark brownish color, suggested the formation of silver nanoparticles (Figure 1). The flasks were observed periodically forchange in color from green to different shades of brown (Table – 1). |
 |
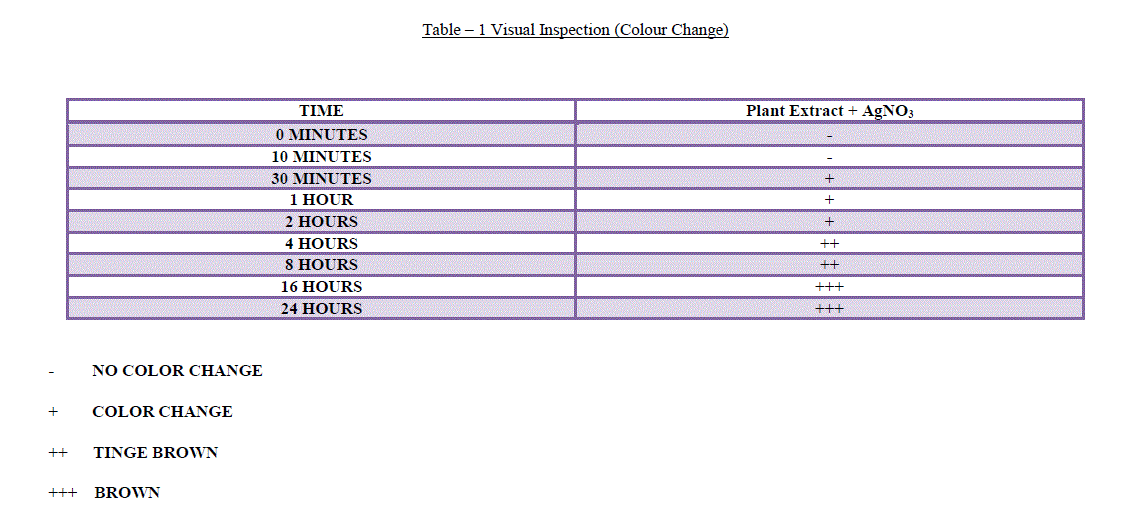 |
| - NO COLOR CHANGE |
| + COLOR CHANGE |
| ++ TINGE BROWN |
| +++ BROWN |
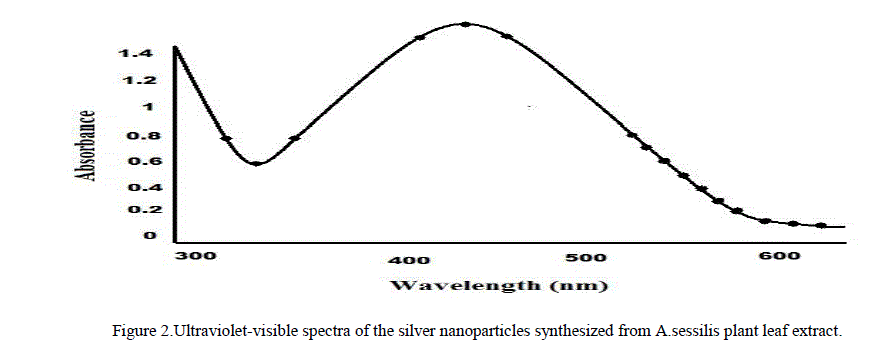
| 2. UV – Visible Spectrum Analysis: This is an important technique to preview the morphology and stability of nanoparticles. The plant mediated silver nanoparticles sample showing an optical absorption band with a peak at about 420 nm typical absorption for metallic silver nano clusters due to the Surface Plasmon Resonance (Figure 2). Plasmon bands are broad with an absorption tail in the longer wavelength. |
 |
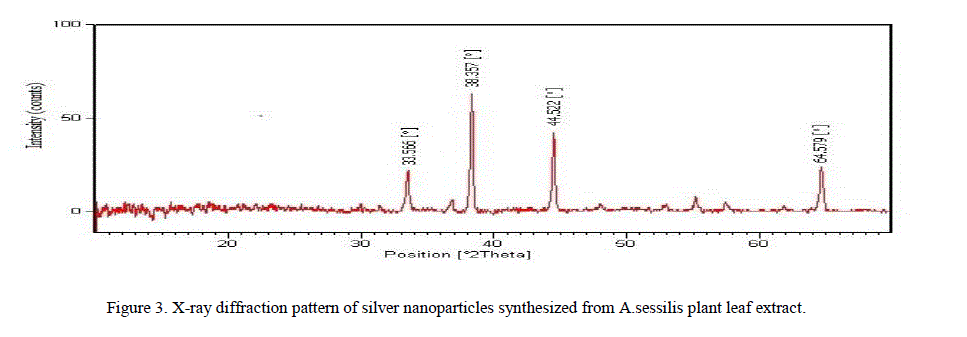 |
| ions respectively revealing that the synthesized silver nanoparticles are composed of pure crystalline silver [13]. |
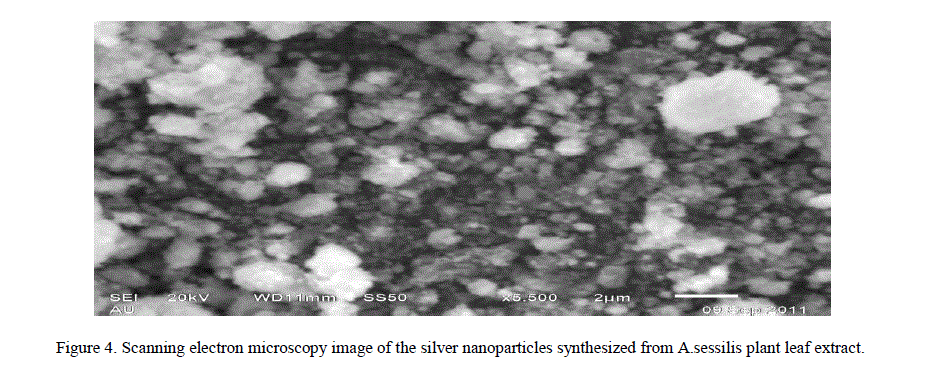
| 4. Scanning Electron Microscopy Analysis: The Scanning Electron Microscopy has been employed to characterization the size, shape and morphologies of formed silver nanoparticles. The SEM images of samples are shown in (Figure 4). From the images, it is evident that the morphology of silver nanoparticles is nearly spherical it is in agreement with the shape of Surface Plasmon Resonance band in the UV – Vis Spectra [14]. The average particle size range analyzed from the SEM images is observed to be 3 nm to 50 nm. Usually biosynthesized silver nanoparticles are covered by biomolecules. In figure, small silver nanoparticles are seen attached to the surface of very large biomolecules. |
 |
| 5. Energy Dispersive Spectroscope Analysis: The additional support of reduction of Ag+ ions to elemental silver, as confirmed by EDS Analysis, showed a peak at 3 keV in the silver region, which confirms the presence of elemental silver as shown. The optical absorption is typical for the absorption of metallic silver nano crystals due to Surface Plasmon Resonance (Figure 5). |
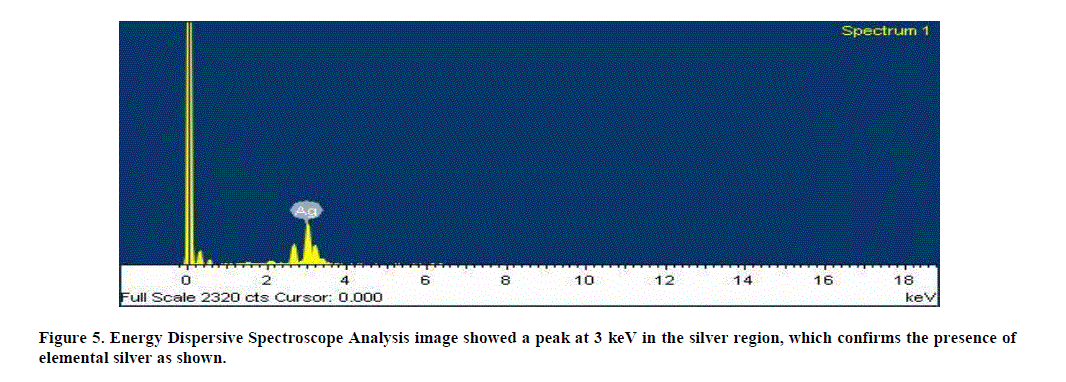 |
| 6. Transmission Electron Microscopy Analysis: The representative TEM picture recorded from the silver nanoparticle film deposited on a carbon coated copper TEM grid has been visualized. Figure showed individual silver nanoparticles as well as number of aggregates. The morphology of the nanoparticles is highly variable; most of the AgNP’S are spherical and are in the range of 3nm to 50nm in size (Figure 6). The nanoparticles were not in direct contact even within the aggregates, indicating stabilization of the nanoparticles by a capping agent [15]. The separation between the silver nanoparticles seen in the TEM image could be due to capping by proteins present in plant material [16]. |
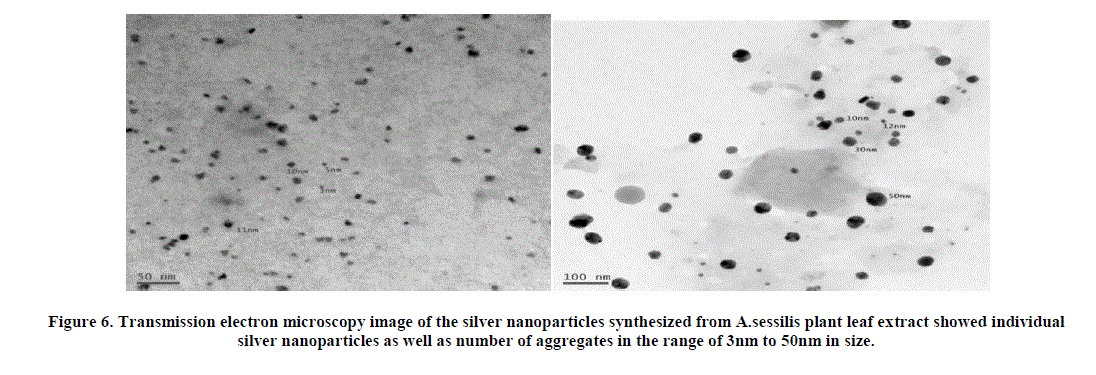 |
| 7. Particle size analysis: The distribution of the particle size was analyzed by particle size analyzer. The mean particle size was 11.9nm and Z–Average was 25.9nm. SEM and TEM images were confirmed that the particles were aggregated and the size measured by the DLS technique may be the size of the cluster rather than an individual particle(Figure 7). |
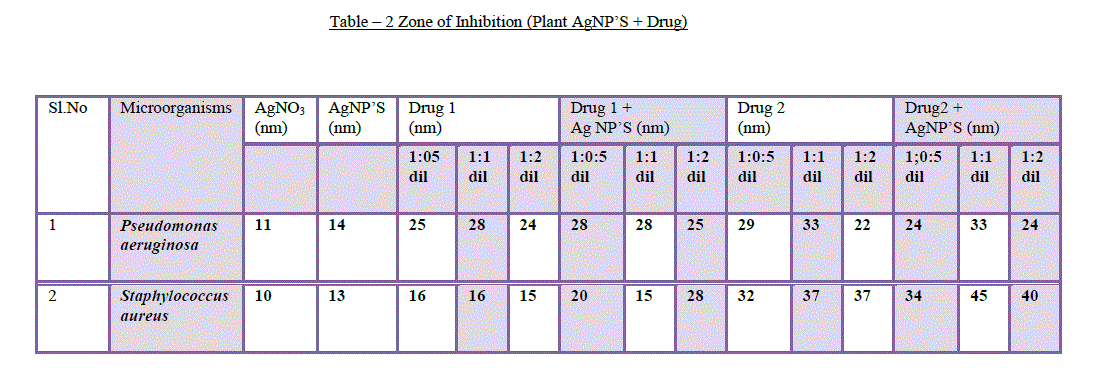 |
 |
| 8. Antibacterial assays: Silver nanoparticles attached to antibiotics exhibited antibacterial properties against human eye pathogens. The antibacterial activity of the synthesized silver nanoparticles along with ocular drugs has been investigated against Staphylococcus aureusandPseudomonas aeruginosa.Sofar, there is no literature available on this method.The silver nanoparticles synthesized by A.sessilis leaf extract are added to Gatifloxacin (drug 1) and Tobramycin (drug 2) in 3 dilutions (1:0.5, 1:1, 1:2 dilutions) and all the three dilutions have shown antibacterial activity against S.aureus and P.aereginosa(Table – 2). Based up on the drug and organism used for antimicrobial testing, the zone of inhibition varies and it depends on synergism [17] and antagonism effect of the drug. Many kinds of ocular drugs control the function of other antibiotic (AgNP’S) when they are applied in combination, this mechanism is antagonism and other kind of drugs showed more activity when they are added with other antibiotics(AgNP’S), this mechanism is synergism [18]. |
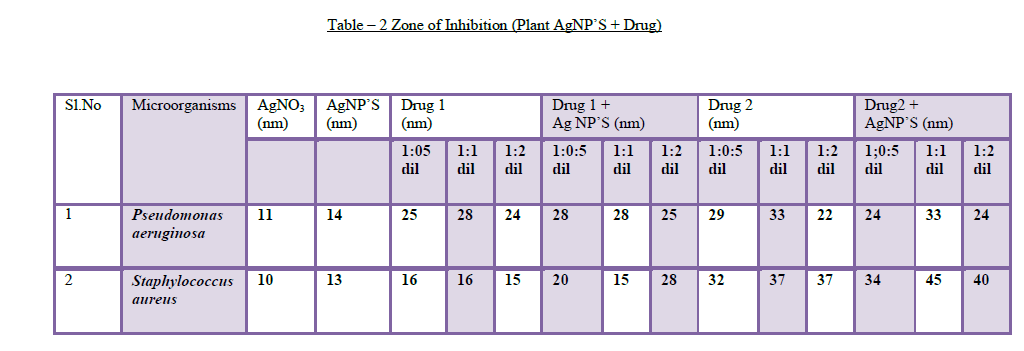 |
| The A.sessilis leaf extract AgNP’S added with drug 1 (Gatifloxacin) showed zone of inhibition against P.aereginosa (28mm, 29mm) with 1:0.5 & 1:1 dilutions and also showed inhibition against S.aureus (20mm, 28mm) with 1:0.5 & 1:2 dilutions. The AgNP’S added with drug 2 (Tobramycin)showed zone of inhibition against P.aereginosa (24mm) dilution with 1:2 and showed synergism effect against S.aureus (34mm, 45mm, 40mm)with 1:0.5, 1:1 and 1:2 dilutions. A.sessilis leaf extract AgNP’S attached to drug 1 and drug 2 showed maximum inhibition against human eye pathogens when they are compared with AgNO3, drug and AgNP’S which are kept as controls (Figure 8). |
 |
| Figure 8.Antibacterial Assays a) The figure showed the maximum zone of inhibition with AgNP’S + DRUG and remaining (AgNO3, AgNP’S, and Drug) showed less inhibition. b) The A.sessilis leaf extract AgNP’S added with Tobramycin showed synergism effect against S.aureus (34mm of zone of inhibition) with 1:1 dilutions. |
V. CONCLUSIONS |
| In this endeavour materials and methods employed, to biologically synthesize silver nanoparticles, are simple, straight and time saving. This novel work involves use of the extract from the leaves of a very commonly and abundantly available plant “A.sessilis” and reduction of silver ions in to AgNP’S. |
| Bioreduction of silver ions is fairly defined and characterized by the following tests: |
| 1. UV-Visible Absorption Spectroscopy (UV-Vis) |
| 2. X-Ray Diffraction (XRD) |
| 3. Scanning Electron Microscope (SEM) |
| 4. Energy Dispersive Spectroscope (EDS) |
| 5. Particle Size Analysis (PSA) |
| 6. Transmission Electron Microscope (TEM) |
| The leaf extract exhibited high degree of reducing and capping properties by forming round shaped silver nanoparticles of 3 to 50 nm size. The process does not involve employment of chemicals so it is safe. The laboratory techniques employed take less time, simple, facile and has economic viability besides being eco-friendly. The nano carrier systems provide prolonged residence time at the ocular surface resulting complete eradication of pathogens. Various bactericidal, wound healing biomedical preparations [19-20] can be designed employing this method. |
| When nanoparticles of silver are attached to antibiotics like Gatifloxacin and Tobramycin, they show maximum zone of inhibition against ocular pathogens such as S.aureus and P.aereginosa. We can expect exceptionally good results from administration of such drugs. Future drug designs may be formulated using this technology to cure different ocular infections in adults and even in neonates. |
| Note: All rights of this article are reserved to Dr. D. Sarvamangala. |