ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
E.Sheeba1, S.Palanivel2 and S.Parvathi2
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
In vitro flowering and an efficient micropropagation protocol was developed for the annual medicinal herb Physalis minima (Solanaceae) by the in vitro culture of nodal segments of mature plant. Murashige and Skoog’s (MS)medium, 3% sucrose and0.8% agar supplemented with 4.0mg/l BAP and 0.25mg/l 2,4 - D and1.0mg/l KIN and 0.25mg/l NAA were found to develop fully functional flowers. MS medium supplemented with 2.0mg/l BAP and 0.25mg/l IAA induced an average of three shoots per explants. Full strength MS medium with 2.0mg/l IBA exhibited the best in in vitro rooting. Seventy percent of the rooted shoots survived when transferred to green house and next to the field condition.
Keywords |
| In vitro flowering, micropropagation, medicinal plant, Physalis minima, Solanaceae. |
INTRODUCTION |
| Physalis minima Linn. is one of the important medicinal plant species belonging to the family Solanaceae. The plants of Physalis minima Linn. are bitter, appetizing, tonic, diuretic, laxative, and useful in inflammations, enlargement of the spleen and abdominal troubles. The fruit is considered as a tonic, diuretic and purgative. Extracts from leaves have antimicrobial activity. The juice of the leaves, mixed with mustard oil and water, has been used as a remedy for earache. The Malays apply the leaves which have been smeared with oil and heated, to ulcers wounds and pustules. The leaves are also used to relieve head-ache. A paste of the leaves and stem is used to treat dizziness. |
| Though the plant has immense medicinal value it is gradually declining from the nature due to over exploitation and environmental pollution [2]. In recent years, numerous studies on in vitro propagation of different plant species have shown that this technique may be a solution for rapid propagation of selected plant species. The present paper reports an efficient micropropagation system for generating a large number of plants and in vitro flowering in Physalis minima. |
MATERIALS AND METHODOLOGY |
A.Plant Materials |
| The plants were collected from Kerala (mainly, Palakkad and Thrissur districts). From the field grown plants, the young nodal region were excised out and used for micropropagation and in vitro flowering. The collected explants were washed thoroughly in running tap water for 30'. Then the explants were rinsed with 1% savlon solution containing 6-8 drops of tween 20 for 15' and again washed with double distilled water to remove the traces of detergent solution. Again, inside the laminar air flow chamber rinse once with autoclaved water. Then the explants washed with 70% alcohol for 30 seconds. Next, the explants rinsed with 0.05% mercuric chloride solution for 5' and again washed with sterile distilled water for 6-8 times. Then the explants were placed in sterile petri plates for inoculation. The sterilized nodal region was used as explant source for in vitro culturing. |
B.Culture medium and Conditions |
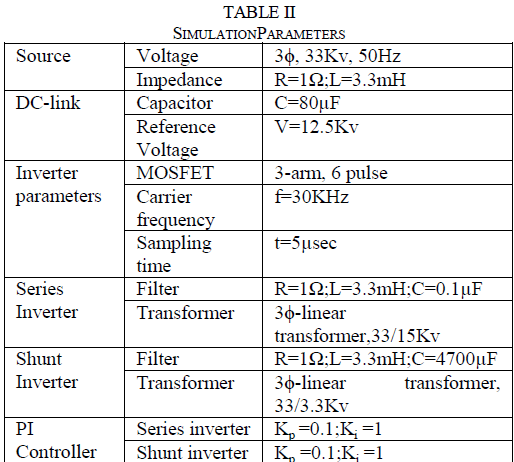
| Basal medium used for initial set of experiments for shoot proliferation. The MS medium (Murashige, T and Skoog, F, 1962) consists of macronutrients,micronutrients,iron source and vitamins supplemented with sucrose(3%) as a carbon source and agar(1%) as a solidifying agent. The pH of the medium was adjusted to 5.8 before autoclaving at a pressure of 15lbs. The basal medium was supplemented with various cytokinins [BAP(6 – Benzyl amino purine) and KIN (kinetin)]( Wang, S; Tan, L and Chen, F, 2001) and auxins [ NAA(α – Naphthalene acetic acid),IAA (Indole – 3 – acetic acid) , IBA (Indole – 3 – butyric acid) and 2,4 – D (2,4 – Dichloro phenoxy acetic acid)] at different concentrations and combinations(Nitsch and Nitsch,J,P, 1967; Sahoo, Y and Chand,P,K,1998 ; Thiruvengadam,M and Narayansamipillai, J, 2000). The cultures were incubated at 25 ± 2oC with 16 hours photoperiod. |
C.Rooting, Acclimatization and Field Transfer |
| The induction of roots was carried out on full strength MS solid medium supplemented with auxin (IBA and NAA) at different concentrations (Vadawale et al, 2006). Healthy shoots with well developed roots were transferred to small pots containing vermicompost at maintained humidity (~ 90%) through misting device inside a greenhouse. |
| Data were collected on number of shoots, shoot length, number of roots, root morphology, number of flower buds and developed flower. |
RESULTS AND DISCUSSIONS |
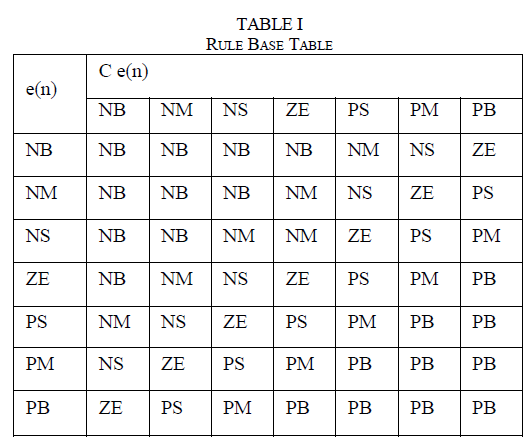
| The explants cultured on MS medium without supplement of growth regulator did not grow at all. MS medium supplemented with different concentrations of BAP in combination with IAA resulted in micropropagation (Table - I). Nodal explants induced an average of three shoots on MS basal medium supplemented with BAP and IAA (Table – I; Fig – Ia).T he maximum percentage of shoot induction was in BAP (2.0mg/l) and IAA (0.25mg/l). |
 |
 |
In vitro flowering |
| The fully developed shoots from the nodal explants isolated and transferred to fresh MS medium having different concentration of cytokinin (BAP or KIN) in combination with auxin (2,4 – D or NAA) started developing flower bud at the nodal region. |
Effect of plant growth regulators on flower induction |
| The effects of cytokinin (BAP or KIN) and the auxin (2, 4 – D or NAA) on flower induction were examined (Table - II). MS medium containing BAP (4.0mg/l) and 2,4 – D (0.25mg/l); KIN (1.0mg/l) and NAA (0.25mg/l) resulted in uniform flowering with 2 – 3 flowers per plant (Fig. Ib). The flower buds induced on the MS medium supplemented with different concentrations of BAP and 2,4 – D developed into functional flower (Fig.Ic), while those flower buds induced on MS medium supplemented with different concentrations of KIN and NAA developed flowers except 4.0mg/l KIN + 0.25mg/l NAA and 5.0mg/l KIN + 0.25mg/l NAA(Sri Rama Murthy et al, 2006). |
 |
Effect of auxin on in vitro rooting |
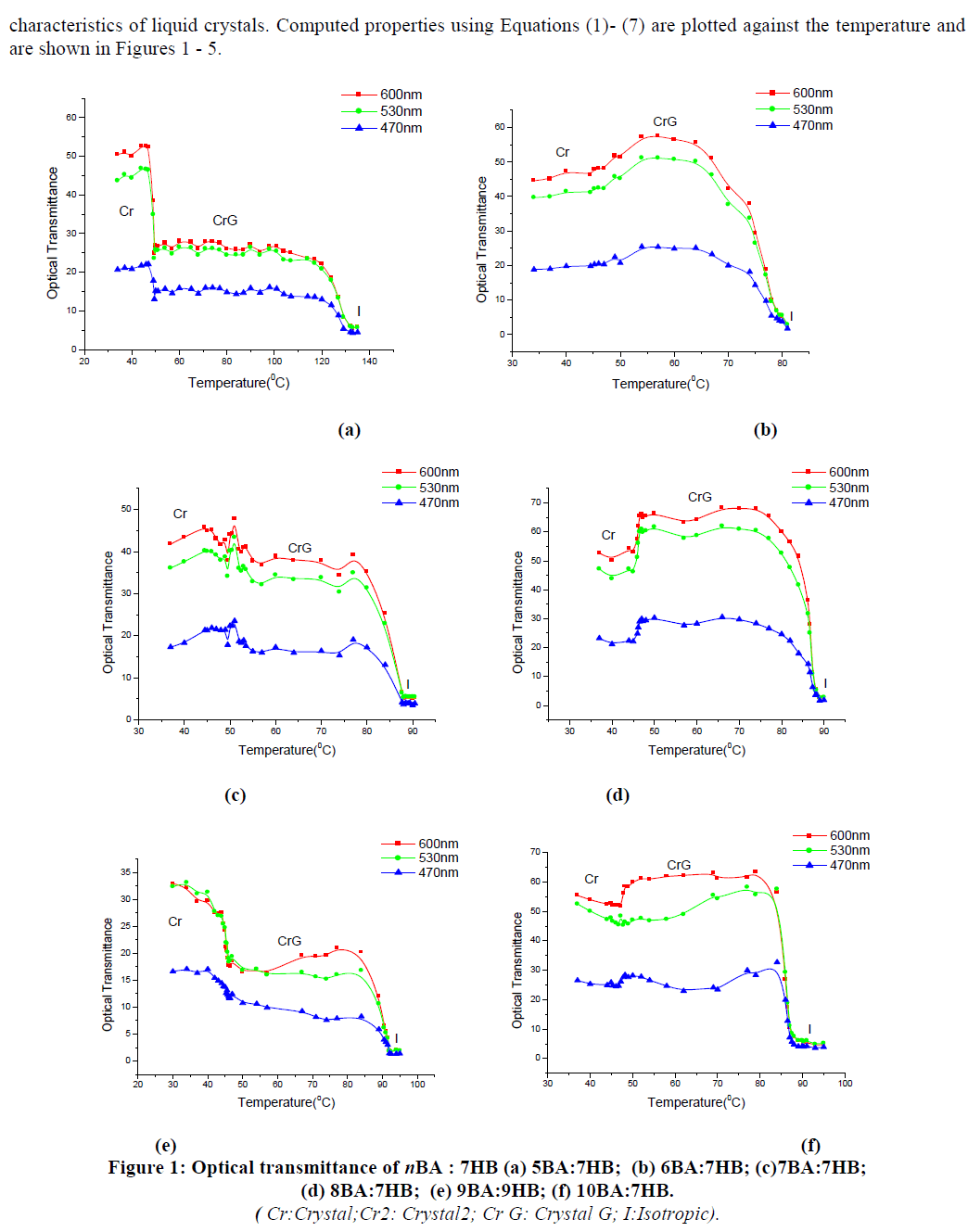
| IBA was found to be more suitable for root induction compared with NAA (Table – III). When shoots were transferred to the rooting medium supplemented with NAA, stout and shorter roots were formed. The regenerated shoots, rooted best on MS medium containing 2.0mg/l IBA (Fig. Id). The roots were thin and with lots of root hairs in the medium with IBA. After three weeks of incubation in the medium with IBA, each shoot developed an average of 5 roots with an average length of 5cm. Slow degradation of IBA facilitates its localization near the site of application and thus, it functions better in inducing roots. |
| Rooted shoots were transferred directly to small pots filled with vermicompost and moss (1:1). Of the plantlets transferred to soil, 70% survived and exhibited morphological characters similar to those of the field grown plants (Fig. Ie). |
 |
CONCLUSION |
| A simple and efficient method has been developed for inducing the in vitro flowering of Physalis minima. In our study, increased in vitro flowering were concentration of cytokinin (BAP or KIN) with less concentration of auxin (2, 4 – D or NAA).The protocol also described for the rapid propagation of this medicinal plant. |
References |
|