ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Jayachamarajapura Pranesh Shubha1, Yadati Madhusudhan2 , Puttaswamy3
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
The kinetics of oxidative decolorization of acid orange 10 (AO10) by chloramine-B and bromamine-B in HClO4 medium has been investigated spectrophotometrically (λmax = 475 nm) at 298 K. Kinetic runs were performed under pseudo first-order conditions of [oxidant]o >> [AO10]o. Under identical experimental conditions, reactions with both the oxidants follow identical kinetics with a first-order dependence on each [oxidant]o and [AO10] and a fractional-order dependence on [HClO4]. Stoichiometry of the reaction was found to be 1:1 and the oxidation products were identified. The reaction was studied at different temperatures and various activation parameters have been computed. Effects of p-toluenesulfonamide, halide ions, ionic strength and dielectric constant of the medium have been investigated. Reaction mixture fails to induce polymerization of acrylonitrile. The rate of oxidation of AO10 is about two-fold faster with BAB as compared to CAB. This may be attributed to the difference in electrophilicities of Cl+ and Br+ ions and also the van der Waal’s radii of chlorine and bromine. Plausible mechanism and related rate law have been deliberated for the observed kinetics.
Keywords |
| Azo dye, acid orange 10, Chloramine-B, Bromamine-B, Oxidation-Kinetics, Acid medium |
INTRODUCTION |
| Variety of dyes used in textile industry usually have a synthetic origin and multifaceted aromatic molecular structures which make them more stable and more complicated to be biodegraded [1-3]. Colored industrial effluent is the most apparent indicator of water pollution and the discharge of highly colored synthetic dye effluents is aesthetically displeasing and cause considerable damage to the aquatic life. The effluents are strongly colored which not only created environmental and aesthetic problems, but also posed a great potential toxic threat to ecological human health as most of these dyes are toxic and carcinogenic. Predominantly azo dyes which contain one or more nitrogen to nitrogen double bonds (-N=N-) constitutes a significant portion that are widely used in industries today. The strong electronwithdrawing character of the azo group stabilizes these aromatic pollutants against conversions by oxygenases. Therefore, azo dyes are not readily degraded under aerobic conditions. Hence, removal of azo dye effluents generated by food and dye industries is a main issue in waste water treatment. These effluents are commonly treated using physico-chemical methods such as adsorption, photo degradation and coagulation. All of these processes are expensive and complicated. Therefore, there is a need for economical and simple methods to abolish harmful dyes in effluents [1- 9]. |
| Acid orange 10 (AO10, orange G) is used in textile fabrics. [1]. Extensive literature survey reveals that there are no reports on the oxidation of AO10 by any oxidants from the standpoint of the kinetic and mechanistic approach. Hence, it was felt advisable to investigate the oxidative decolorization of AO10 with N-haloamines to explore the kinetic and mechanistic aspects of its redox chemistry. |
| The most important aim of this work was to promote the decolorization of AO10 by N-haloamine, has a low energy cost and is economical. The effectiveness of the proposed process was evaluated by its capability to promote decreases in color and total organic carbon content. The high efficiency observed with the dye model showed that this economic, easily operated and maintained treatment process could also be employed in the remediation of effluents. |
| The chemistry of a class of N-metallo-N-haloarylsulfonamides, known as N-haloamines, attracted the attention of many investigators due to their diverse behaviour. Their versatile nature is attributed to their ability to act both as bases and nucleophiles [10]. As a result of this, these compounds interact with a wide range of functional groups in aqueous, partially aqueous and non-aqueous media in presence of acids or alkalis, bringing about an array of molecular transformations. In general monohaloamines undergo two electron change while dihaloamines act as four electron oxidants. The reduction products obtained are the respective sulfonamide and sodium chloride [11]. The dominant members of this class of chlorocompounds are chloramine-T (CAT) and chloramine-B (CAB). A review of literature reveals that although the reaction of aromatic sulfonyl chloramines have been known and extensively investigated [10, 12-16] there is not much of information [17-20] available on the reaction of corresponding bromamines, bromamine-T and bromamine-B. Sodium N-bromobenzenesulfonamide or bromamine-B (BAB) has gained importance as a mild oxidant and it can be readily prepared by brominating CAB. Bromamine-B is found to be a most potential oxidant among these N-haloamines. There are but a few reports [21-22] on the kinetics of oxidation of organic substrates by BAB as compared to the studies with other haloamines as oxidants from mechanistic view point. For these reasons, it was felt interesting to investigate the mechanism of oxidation of AO10 with this reagent. |
| In the light of existing information and in continuation of our research interest on the kinetic and mechanistic investigations of oxidation of various substrates in general and dyes in particular by CAB and BAB, the title reaction was undertaken. Accordingly, in this communication we report on the comprehensive kinetics of AO10 oxidation by CAB and BAB in HClO4 medium at 298 K. |
EXPERIMENTAL |
| Chloramine-B (E. Merck) and acid orange 10 (Sigma Aldrich) were used as received. Bromamine-B was prepared [23] by the partial debromination of dibromamine-B (DBB) as follows. Pure chlorine was bubbled through an aqueous solution of chloramine-B (30 g in 560 mL water) and liquid bromine (6 mL) was added dropwise with constant stirring. A yellow precipitate of DBB formed was washed well with H2O, filtered under suction, and dried in a vacuum desiccator. Dibromamine-B (31.5 g) was digested in batches with constant stirring in 50 mL of 4 mol dm-3 NaOH. The resulting mass was cooled in ice, filtered under suction, and the product (BAB) was dried over anhydrous calcium chloride. The purity of BAB was tested iodometrically through its active bromine content and its FT-IR spectrum. Aqueous solutions of BAB were prepared, standardized whenever required by the iodometric method and preserved in brown bottles to prevent its photochemical deterioration [24] |
| Solvent isotope studies were made with D2O (99.4 %) supplied by BARC, Mumbai, India. Analytical grade chemicals and double distilled water was used throughout. |
| Kinetic measurements |
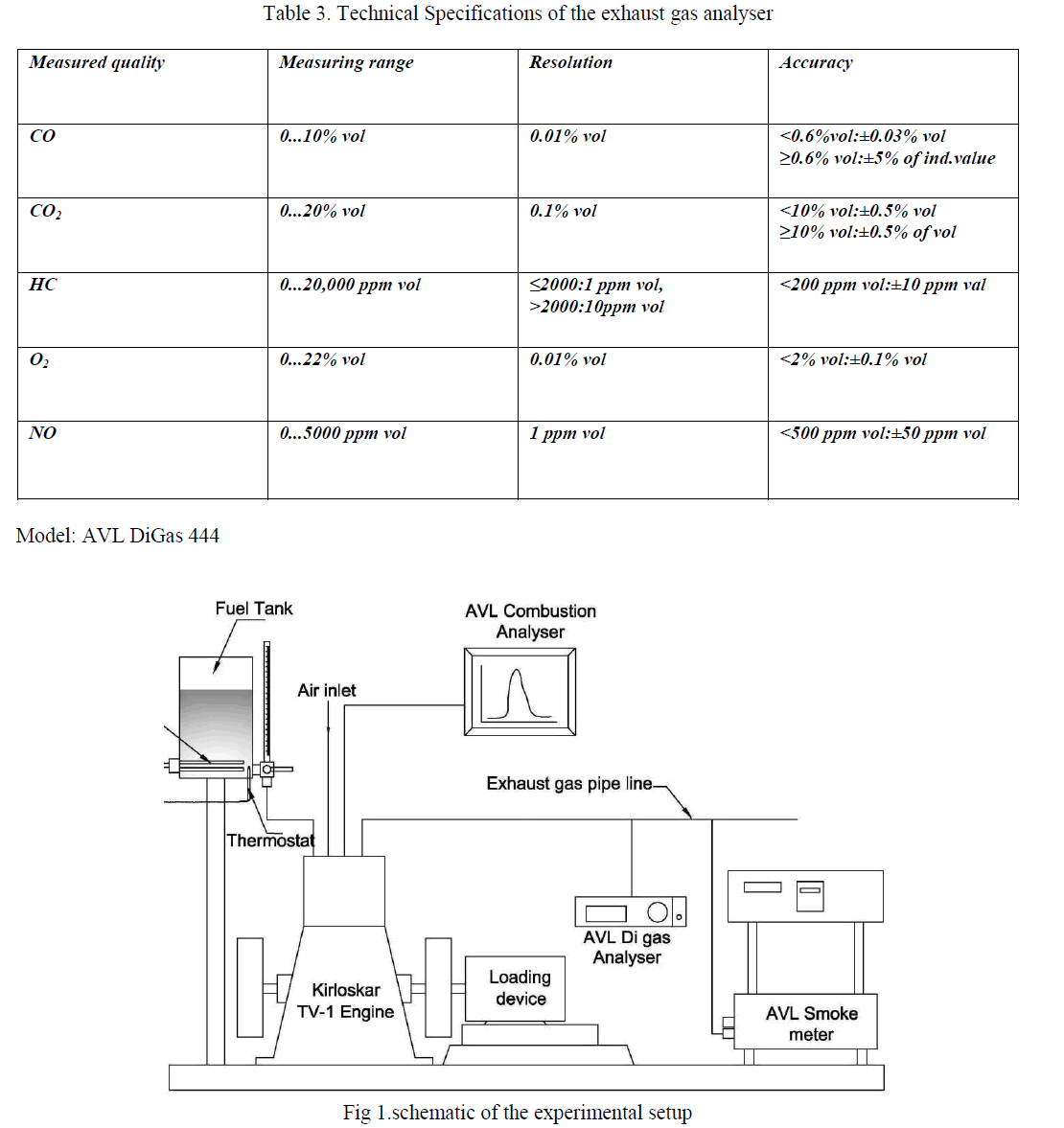
| Kinetic measurements were carried out using a UV–Visible spectrophotometer (Elico SL159). In the present study, the kinetic experiments were carried out between 288 and 308 K. For this purpose, a Raagaa Ultra Cold Chamber with digital temperature control (Chennai, India) was used. The temperature was maintained constant with an accuracy of ± 0.1 0C. Detailed kinetic runs were performed under pseudo first-order conditions of [oxidant]0>>[AO10]0 at 298 K. Reactions were conceded in glass stoppard pyrex boiling tubes whose outer surfaces were coated black to prevent photochemical effects. The oxidant as well as the requisite amounts of AO10, HClO4 solutions and water (to keep the total volume constant for all runs) taken in separate tubes were thermostatted for 30 min at 298 K. The reaction was initiated by the rapid addition of a measured amount of oxidant to the stirred reaction mixture. Instantaneously, 4 cm3 of the solution was pipetted into a cuvette placed in the UV-Vis spectrophotometer and absorbance measurements were made at 475 nm (λmax for AO10) for more than two half-lives. The absorbance readings at t = 0 and t = t are D0 and Dt. Plots of log D0/Dt versus time were made to evaluate the pseudo first-order rate constants (k/) which were found reproducible within ±4–5%. |
 |
 |
RESULTS AND DISCUSSION |
| Effect of reactant concentration on the rate |
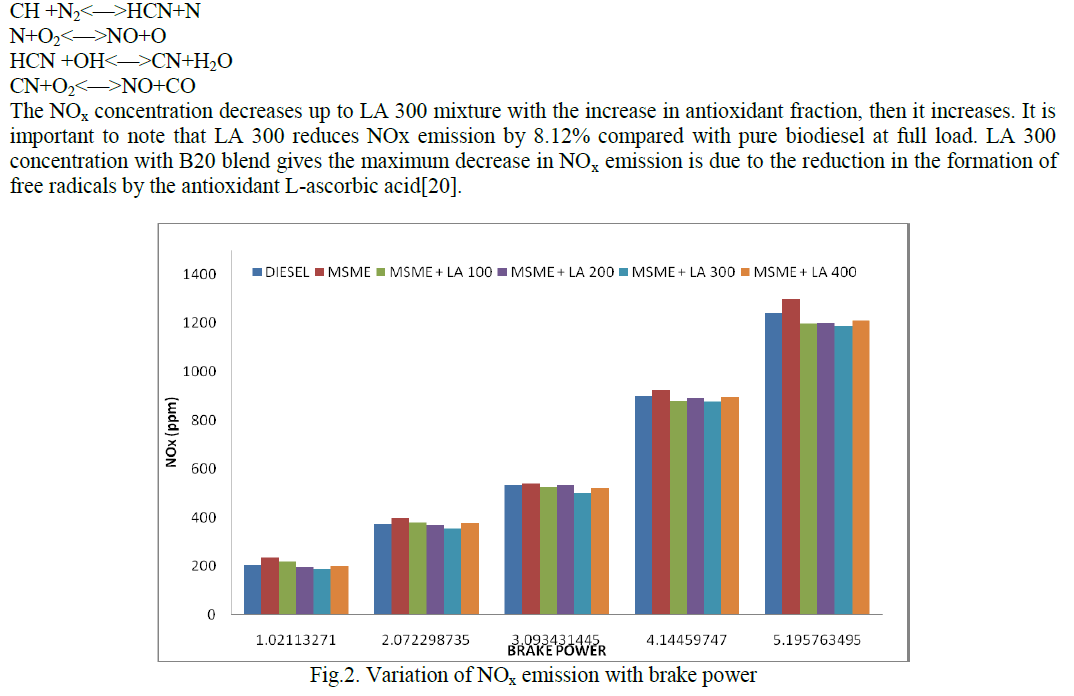
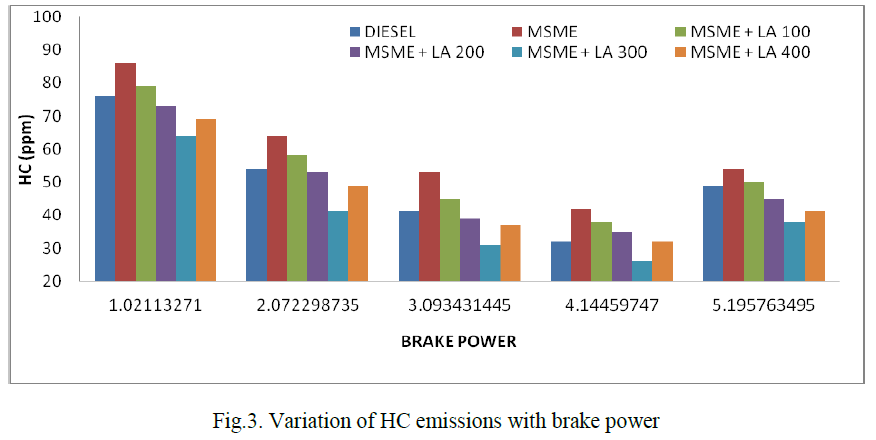
| The kinetics of oxidation of AO10 by CAB and BAB (hereafter condensed as oxidant) have been investigated at several initial concentrations of the reactants, under pseudo first-order conditions of [oxidant]o >> [AO10]o, in presence of HClO4 at 298 K in both cases. The kinetic and mechanistic features for the oxidation of AO10 with the intimately related compounds CAB and BAB in HClO4 medium are same under identical experimental conditions but the comparative rates of oxidation of AO10 by BAB are about two-fold faster than CAB. |
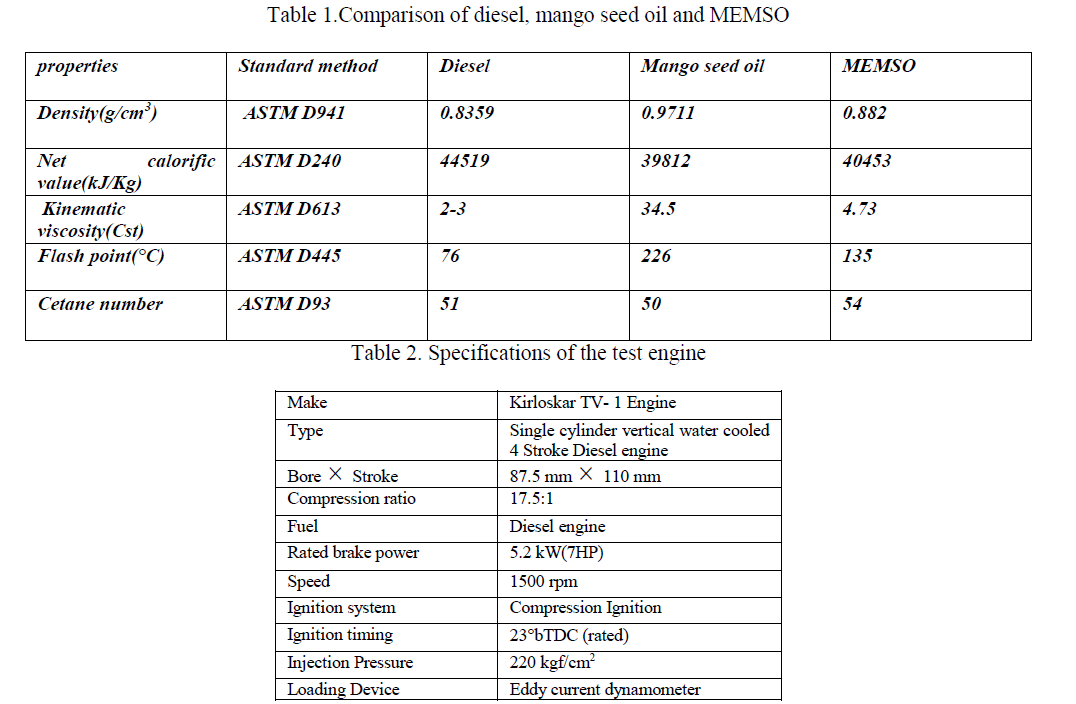
| Under the conditions [oxidant]0 >> [AO10]0 at constant [oxidant]0, [HClO4], temperature, plots of log (absorbance) versus time were linear (r>0.9972) indicating a first-order dependence of rate on [AO10]0 in both the cases. The linearity of these plots in both cases, together with the constancy of the slopes obtained at different [AO10]0, substantiates the first-order dependence of rate on [AO10]0. The pseudo first-order rate constants (k/) obtained are recorded in Table 1. |
 |
 |
| Fig. 5 Plot of log [HClO4] versus log k/. |
| Effects of halide ions and benzenesulfonamide concentration on the rate |
| Addition of halide ions, Cl- or Br-, in the form of their sodium salts (1.0 x 10-3 - 8.0 x 10-3 mol dm-3) showed no pronounced effect on the rate. This indicates that the halide ions play no role in the reaction. The ionic strength of the reaction medium was varied from 0.1 to 0.3 mol dm-3 with NaClO4 solution keeping other experimental conditions constant. It was found that addition of NaClO4 showed negligible effect on the reaction rate, representing the participation of nonionic species in the rate-determining step. Hence no attempts were made to maintain the ionic strength of the medium stable for kinetic runs. Addition of benzenesulfonamide (RNH2) to the reaction mixture (5.0 x 10-3 mol dm-3) did not influence the rate significantly indicates that RNH2 is not involved in any step prior to the rate determining step of the proposed scheme. |
| Effect of dielectric constant of the medium and solvent isotope on the rate |
| The dielectric constant (D) of the medium was mottled by adding MeOH (0-30 % v/v) to the reaction mixture with all other experimental conditions being held constant but the rates were not considerably altered with both the oxidants. Since the oxidation of AO10 by CAB and BAB was increased with H+ ions, the solvent isotope effect was studied in D2O as the solvent medium for both the oxidants. The rate constants for CAB and BAB revealed that k/ (H2O) was equal to 3.58 x 10-4 s-1 and 7.52 x 10-4 s-1, and k/ (D2O) was 4.16 x 10-4 s-1 and 10.6 x 10-4 s-1, respectively. Thus, the solvent isotope effect, k/ (H2O) / k/ (D2O) were found to be 0.85 and 0.70 for CAB and BAB. |
| Effect of temperature on the rate |
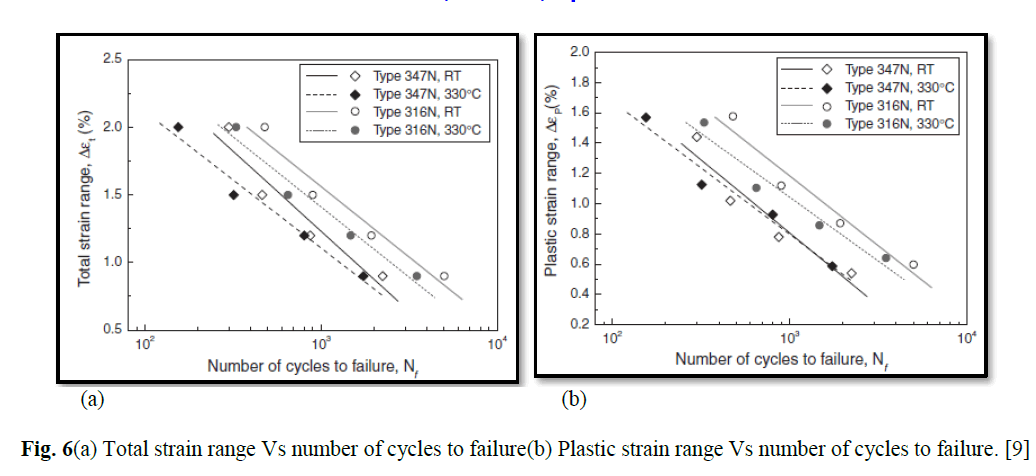
| The reaction was studied at different temperatures (288-313 K), keeping other experimental conditions constant. From Arrhenius plots of log k/ vs. 1/T (Fig. 6; r > 0.9955), composite activation parameters (Ea, ΔH≠, ΔS≠, ΔG≠ and log A) were computed for the oxidation of AO10 by CAB and BAB. These data are summarized in Table 2. |
 |
 |
 |
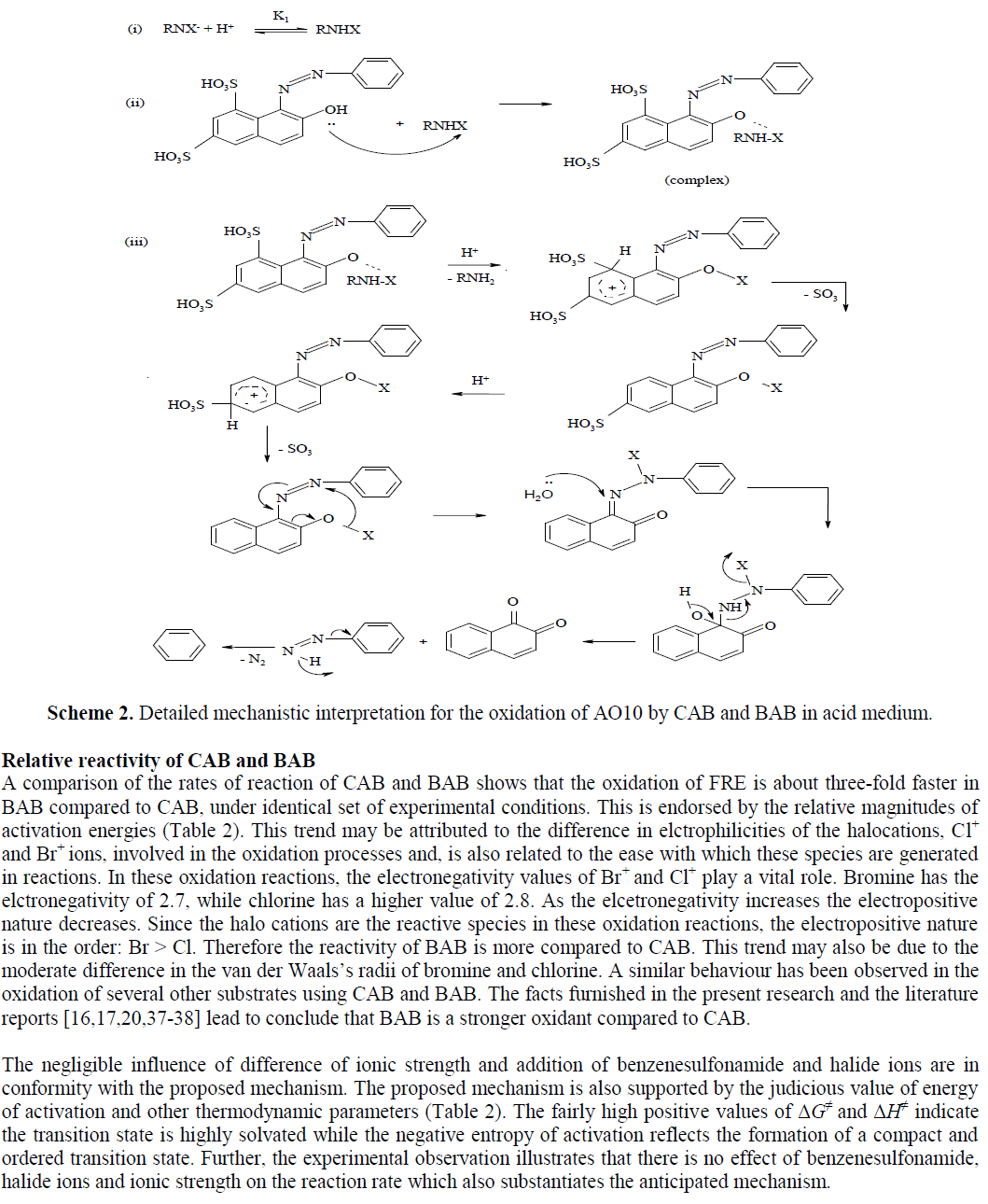 |
| In the present redox system the optimum conditions for the controlled oxidation of AO10 by CAB and BAB to naphthalene and 1,2-naphthaquinone in acid medium have been developed. Accordingly, this redox system can be scaled up to industrial operation. Furthermore, AO10 is one of the chief components in the effluents of various industries and is environmentally hazardous and also carcinogenic compound. Hence, the present simple method developed can be adopted for treating the AO10 dye present in industrial effluents to reduce toxicity caused by this dye. Also, this method offers several advantages including short reaction time, cost effective and moderately non-toxic reagents which make the reaction process simple. |
CONCLUSION |
| In the present work, optimum conditions for the oxidative decolorization of AO10 by CAB and BAB in acid medium have been obtained. Chloramine-B decolorizes AO10 almost completely in 55 minutes in acid medium whereas bromamine-B takes hardly 40 minutes. The kinetics of oxidation of AO10 by CAB and BAB in acid medium obeys the rate law –d[oxidant] / dt = k [oxidant]o [AO10]o [H+]x, where x= 0.76 and 0.65 for CAB and BAB respectively. Hence this method is simple and efficient method including a number of advantages such as cost effective, short time, use of relatively non-toxic reagents. Furthermore, the simple and well-designed method developed in the present research can be implemented with suitable alteration for treating AO10 present in industrial effluents to diminish the toxicity caused by this dye. In addition to this the kinetic and mechanistic picture of AO10-CAB/BAB redox system in acid medium has also been elucidated. |
ACKNOWLEDGMENTS |
| The authors are thankful to The Principal and The Management, Don Bosco Institute of Technology for the facilities and support. Financial assistance from Visvesvaraya Technological University Research Grants, NO VTU/Aca./2012- 13/A-9/760 is gratefully acknowledged. |
References |
|