ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
S. J. Varjani1, D. P. Rana2, S. Bateja3, M. C. Sharma4 and V. N. Upasani5*
|
| Corresponding Author: vnu_halophiles@yahoo.com |
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Sixty nine hydrocarbon utilizing bacteria isolated from selected crude oil contaminated sites of ONGC (Gujarat) were screened for biosurfactant producing potential. VSHUB005 showing 28.59 ± 0.50% emulsification index, 69.73 ± 0.53 mN/m surface tension and 90.46 ± 0.08% interfacial tension reduction was considered as potential biosurfactant producer using primary (hemolytic activity and CTAB agar plate method) secondary (drop collapse, oil spread, emulsification index and surface tension) and tertiary (interfacial tension) screening methods. Very strong, negative correlation between surface tension measurement and interfacial tension reduction (rs= -0.961, Æ = -1.000) was measured. VSHUB005 was identified as P. aeruginosa by 16S rDNA gene sequencing technique. The GenBank accession number for 16S rDNA sequence and NCIM culture deposition ID assigned are KC713611 and 5514, respectively
Keywords |
| Biosurfactant; Drop collapse method; Oil spread method; E24, Surface tensin, Interfacial tension; VITEK®2 Compact |
INTRODUCTION |
| Petroleum and oil industry is one of the most important sectors in India. The pollution caused by this industry due to natural or anthropogenic activities damage terrestrial and aquatic systems. The poor bioavailability, hydrophobicity and low aqueous solubility of these petroleum pollutants limit their biodegradation by microorganisms [1,2]. Surfactants are found to enhance degradation and recovery of crude oil. Surfactants and emulsifiers which may be synthetic or of biological origin are inseparable components of daily life [1,3]. Surfactants are amphiphilic molecules possessing wide variety of surface activity. As they are of both hydrophilic and hydrophobic in nature they reduce surface and interfacial tensions by accumulating at the interface by formation of micelle between two immiscible fluids like oil and water and facilitate hydrocarbon uptake and emulsification [4-6]. Depending upon their charge they are classified as anionic, cationic and non-ionic. They are widely used in various industries, such as pharmaceuticals, cosmetics, petroleum, food, etc. The drawback of chemical surfactants of petroleum origin that are used in different industries is their toxicity, non-biodegradability and costly manufacturing processes [3,7]. Naturally produced surface-active compounds of biological origin are called biosurfactants (bioemulsifier) (BS (BE)) [8]. During last decade there has been an increased interest from the scientific and industrial community towards microbial surfactants due to their lower toxicity, higher biodegradability i.e. ecofriendly nature, higher foaming capacity, diversity, possibility of large scale production and optimal activity at extreme conditions of temperatures, pH levels and salinity [4,6]. Biosurfactants are broadly classified as low molecular weight (glycolipids, lipopeptides) or high molecular weight (polysaccharides, proteins) molecules [9]. BS with diverse chemical structures and properties are produced by various microorganisms isolated from petroleum contaminated sites [1,10,11]. There are numbers of reports available for BS production by microorganisms using water insoluble substances such as crude oil, diesel, crude oil or oily sludge [12] and water-soluble compounds such as glucose, sucrose, ethanol or glycerol as substrates [10]. Biosurfactants have many important applications in petroleum-related industries i.e. use in enhanced oil recovery, cleaning oil-contaminated water/soil, viscosity control, oil emulsification and removal of crude oil from sludges [12-14]. The diverse applications of BS have lead to the development of rapid and reliable methods to screen for efficient BS producing bacteria. BS production is detected by various techniques like Hemolytic activity (HA)/ Blood agar method [5,15], N-Cetyl-N,N,N-trimethylammoniumn bromide (CTAB) agar plate method [16], cell surface hydrophobicity techniques viz. bacterial adherence to hydrocarbons (BATH) assay [11] and microbial adherence to hydrocarbons (MATH) assay [17], drop collapse method (DCM) [18,19], oil spreading method (OSM) / oil displacement method [20], emulsification index (EI) (E24) [21], emulsification assay (EA), Tilted glass slide (TGS), hydrocarbon overlay agar (HOA) [22], Aximetric drop shape analysis (ADSA), Direct colony-thin layer chromatographic, Turbidity assay [23], Surface activity/ Tensiometric measurement both surface tension (SFT) [24] and interfacial tension (IFT) [25]. HA is often used for preliminary screening of microorganisms for ability to produce BS on hydrophilic media [26]. This research paper deals with screening for BS producers from sixty nine (69) hydrocarbon utilizing bacteria (HUB) by the following methods: hemolytic activity (HA), N-Cetyl-N,N,N-trimethylammonium bromide (CTAB) agar plate method, drop collapse method (DCM), oil spread method (OSM), emulsification index (E24), surface tension (SFT) and interfacial tension (IFT). We have also tried to compare/evaluate these methods for screening purpose. This is the first report where IFT measurement was used as a part of screening methodology for BS (BE) production. Apart from screening, this study also analyzes the credibility of the use of hydrophobic and hydrophilic substrates like crude oil, glucose and glycerol for the growth of BS (BE) producing bacteria isolated from crude oil contaminated environment. |
II. MATERIALS AND METHODS |
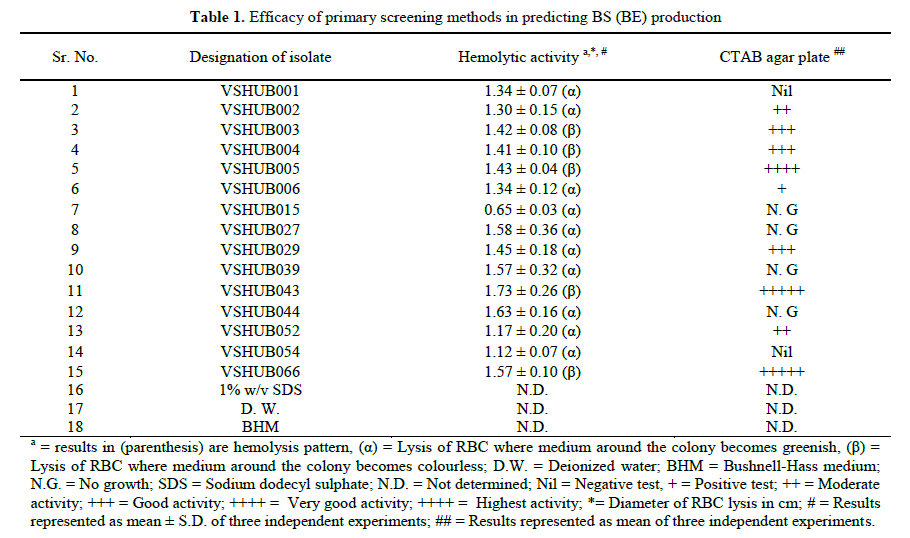
| 2.1. Bacterial isolates: Sixty nine hydrocarbon utilizing bacterial (HUB) isolates used in this study were isolated from various ONGC oil fields located in Ankleshwar and Ahmedabad assets. Enrichment, isolation and qualitative crude oil utilization/degradation is described elsewhere [14,27,28]. 2.2. Culture medium and bacterial growth: Bacterial isolates were grown in Bushnell-Haas medium (BHM) supplemented with 1% v/v crude oil and incubated for 24 h at 37 ± 1ºC at 180 rpm. Flasks containing sterilized BHM supplemented with Crude oil (1% v/v) (K#X), Glucose (1% w/v) and Glycerol (1% w/v) were inoculated with activated bacterial culture (1% v/v) and incubated for 96h at 37 ± 1ºC at 180rpm. After 96 h the culture broth was centrifuged at 10,000g for 10min to remove cells and other metabolites. The cell free supernatant served as the source of crude biosurfactant. This cell free supernatant was used for DCM, OSM, E24, SFT and IFT measurements, whereas bacterial cells were used for HA and CTAB agar method. All the screening experiments were performed in triplicates and the mean ± s.d. values were used as results. 2.3 Screening for biosurfactant (bioemulsifier) production Initially sixty nine (69) isolates were preliminary screened for BS (BE) production by the primary screening methods namely hemolytic activity (HA) and CTAB agar plate method. Fifteen (15) isolates selected were repeatedly screened by same methods. 2.3.1. Primary screening 2.3.1.1. Hemolytic activity (HA): HA was performed using 5% (v/v) human blood agar plate (s). 50μl of activated bacterial culture was spot inoculated on to blood agar plates and incubated for 48h at 37°C. The plates were visually inspected for zone of clearance (hemolysis) around the colony. The diameter of the zone of clearance is a quantitative method used as an indicator of BS production. Hemolysis was designated as α (alpha), β (beta) and γ (gamma) hemolytic activity, which served as a qualitative method [5,15,22]. 2.3.1.2 CTAB agar plate method:CTAB agar plate method is a semi-quantitative assay for the detection of extracellular glycolipids or other anionic surfactants [6,16]. Blue agar plates were prepared using BHM supplemented with CTAB (20mg/100ml, cationic surfactant), methylene blue (0.5mg/100ml, basic dye) and glucose (2% v/v). Anionic BS producing colonies on blue agar plate were identified following the formation of dark blue halos around the colonies on a light blue plate background. BS producing cultures were scored ranging from “+” to “+++++” corresponding from positive to complete dark blue halo formation [16,22]. 2.3.2. Secondary screening 2.3.2.1. Drop collapse method (DCM): 20μl of cell free supernatant and 20 μl of each control (negative control: distilled water, positive control: 1% w/v SDS) solution were placed to the hydrophobic surface of parafilm with the help of syringe at an angle of 45º. DCM for BHM was also performed to check effect of BHM on drop collapse of distilled water. After 1 min the drops were examined visually under visible light. The syringe was rinsed three times with distilled water and three times with alcohol between each measurement. The shape of the drop on the surface was inspected after 1 min. Result was considered positive for BS production when the drop diameter was at least 1mm larger than that produced by distilled water (negative control). BS producing cultures gave flat drops with scoring system ranging from “+” to “++++” corresponding from partial to complete spreading on the oil surface. Aliquots from a culture of bacterial isolates were analyzed on separate surface of parafilm. [5,18,29]. 2.3.2.2. Oil spread method (OSM): 10μl of crude oil (K#X) was put onto the surface of 40 ml of distilled water in a petri-dish (150 mm in diameter) to form a thin membrane. 10μl of cell free supernatant and 10 μl of each control (negative control: distilled water, positive control: 1% w/v SDS) was gently put onto the center of the oil membrane. OSM for BHM was also performed to check effect of BHM on oil spread of distilled water. Micropipette was rinsed three times with distilled water and three times with alcohol between each measurement. If BS is present in the cell free supernatant, the oil will be displaced with an oil free clearing zone and diameter of this clearing zone indicates the surfactant activity, also called oil displacement activity. A negative control was maintained with distilled water (without surfactant) and BHM, in which no oil displacement or clear zone was observed and 1% w/v SDS was used as the positive control. Area of clearly formed oil displaced circle was measured as the activity of surfactants. The results were scored ranging from “+” to “++++” corresponding from partial to complete displacement of crude oil. The oil displaced area formed by the activity of surfactants showed linear relations to the amount of surfactants tested [20]. 2.3.2.3. Emulsification index (EI) (E24): The emulsification index (E24) was measured using the method described by Cooper and Goldenberg [21]. This method was used to check the stability of the BS extracted. BS activity was measured by adding 6 ml of kerosene to 4 ml of cell-free supernatant. The mixture was vortexed at high speed for 2 minutes. The height of emulsion was measured by taking the layer formed in between aqueous and kerosene layer. Measurement was taken after 24 hours. Emulsions formed by the isolates were compared to those formed by distilled water as control. E24 was calculated at 25â°C. The emulsification index was determined using the following formula. Emulsification index (E24) (%) = (Height of Emulsion layer) ×100/(Total height) 2.3.2.4. Surface tension (SFT) measurement: SFT is a property of the surface of a liquid that causes it to behave as an elastic sheet [30]. Measurement of SFT using a tensiometer is one of the common methods to screen BS producing organisms. SFT of the aqueous solution (distilled water as control and respective cell free supernatant for each culture) was measured by using a Du Nouy ring type tensiometer (Model: OSK A-1274, Ogawa Seiki Co. Ltd., Tokyo, Japan). SFT for BHM was also measured to check effect of BHM on SFT of distilled water. The SFT measurement was carried out at 20 ºC after dipping the platinum ring (4cm diameter) in the solution for a while in order to attain equilibrium conditions. The ring hanging from the balance hook was immersed into the liquid. Then, the ring was slowly pulled up, by lowering the sample plate. Finally, the force applied on the ring while pulling through the surface is recorded. The measurement was repeated three times, platinum ring was rinsed three times with distilled water and three times with alcohol between each measurement. If BS is present in the cell free supernatant, the reading on vernier will decrease than that for distilled water (without surfactant) and BHM. The isolates for which SFT ≤ 47 mN/m was noted were selected for tertiary screening [5,6,17,30]. 2.3.3. Tertiary screening IFT measurement: The IFT was determined by using a spinning drop video tensiometer (Model: SVT 15, Data Physics, USA). The instrumental details are explained elsewhere [31]. Cell free supernatant was loaded into glass capillary followed by injection of 1.5 ml cell free supernatant and required amount of crude oil to form crude oil droplet. The glass capillary with solution was spun in the tensiometer and IFT was determined from the crude oil drop geometry. Data was collected after spinning and analysis was done. IFT was measured at 70ºC. Results were noted as % decrease of IFT by individual culture supernatants using distilled water as control. IFT for BHM was also measured to check effect of BHM on IFT of distilled water [25]. 2.4. Statistical analysis: SPSS for Windows, version 16.0 and Microsoft Excel 2007 were used for the statistical evaluation and graphical representations of results for various methods used to screen for BS producers. All analysis were performed in triplicates and represented as mean or mean ± standard deviation (s.d.). Furthermore, statistical correlations were calculated using Pearson’s correlation coefficient and Spearman rank correlation coefficient (p = 0.01, 2-tailed). The correlation coefficient ranged between -1.000 (strong negative correlation) to 1.000 (strong positive correlation) [32,33]. 2.5 Characterization and identification of BS (BE) producing isolate: Morphological characteristics were studied by performing Gram staining and cultural characteristics were recorded from Nutrient agar (N. agar) plate. VSHUB005 was identified by VITEK®2 Compact (BioMerieux, Inc., USA) at Supratech Micropath Laboratory, Ahmedabad, Gujarat. This is a totally automated system for ID and AST with MIC values as per CLSI (Clinical and Laboratory Standards Institute) guidelines. Isolates were sub-cultured on nutrient agar medium and incubated for 24h at 37°C before testing [34]. Molecular identification of VSHUB005 was performed by amplification of 16S rDNA. Genomic DNA was isolated from culture by using a commercial kit (GenElute Bacterial Genomic DNA Kit, Sigma, USA). Polymerase chain reaction was carried out using the universal primers viz. forward primer (FDD2: CCGGATCCGTCGACAGAGTTTGATCI) and reverse primer (RPP2: CCAAGCTTCTAGACGGITACCTTGTTACGACTT). The identification of phylogenetic neighbors was initially carried out by the BLASTN program against the database containing type strains with validly published prokaryotic names and representatives of cultured phylotypes. Phylogenetic tree (neighbour-joining analysis) and data matrix were performed by MEGA 5.2 software [35- 39]. The 16S rDNA gene sequence and bacterial culture were submitted to GenBank and National Collection of Industrial Microorganisms (NCIM at NCL, Pune), respectively. |
III. RESULTS AND DISCUSSIONS |
| Microorganisms present in oil polluted environments produce various bioactive compounds. Among these BS (BE) has attracted major interest and attention due to their structural and functional diversity. Some of the bacterial species producing BS (BE) commonly found in such environments, include representatives of the genera Bacillus, Halomonas and Pseudomonas [22]. Effective screening methodologies and improved purification techniques are essential in order to achieve desired quantities and qualities of BS (BE) [22]. With these concerns, we have carried out comparative studies on various screening methods for BS (BE) production on hydrocarbon utilizing bacterial isolates. 3.1. Primary screening: The lysis of human red blood cells has been recommended as a simple, easy and primary screening method to test for BS activity. It is also reported that all BS do not have a HA, and compounds other than BS (lytic enzymes) may cause hemolysis [12,15,40]. Out of 69 isolates 15 (21.73%) and 44 (63.76%) isolates showed diameter of zone of hemolysis greater than and less than 0.5 cm, whereas hemolytic activity was negative for 10 (14.49%) isolates. From 15 isolates which showed diameter of zone of hemolysis greater than 0.5 cm, 10 (66.66%) and 5 (33.33%) isolates showed α-hemolysis and β- hemolysis, respectively (Table 1). It has been previously reported that blood agar lysis test gives high percentage of either falsenegatives or false-positive results. 16% false positive results for HA were obtained by Youseff et al., the reason for false results could be virulence factor or poor diffusion of BS in blood agar plate [32]. Another drawback of HA is that hydrophobic substrates cannot be included as sole carbon source in this assay [6]. Afshar et al. suggested HA is not a reliable method for screening for BS producers [33]. Whereas Mulligan et al. recommended the HA as a preliminary screening method which should be supported by other techniques based on surface activity measurements [15]. CTAB agar plate (methylene blue and BS complexation) method was reported as a new, rapid, easy, systematic, non-tedious, non-laborious method for BS detection which could be transferred to liquid culture conditions [6,11]. Positive results were noted as dark blue halos around the colonies on a light blue plate background. Out of 69 isolates 25 (36.23%) isolates showed dark blue halo, 13 (18.84%) isolates did not show dark blue halo indicative of anionic BS production and cationic BS production. Results for CTAB agar test are represented in Table 1.The disadvantage of this method is that CTAB is harmful and inhibits the growth of some microbes and CTAB could be replaced by another cationic surfactant [6]. |
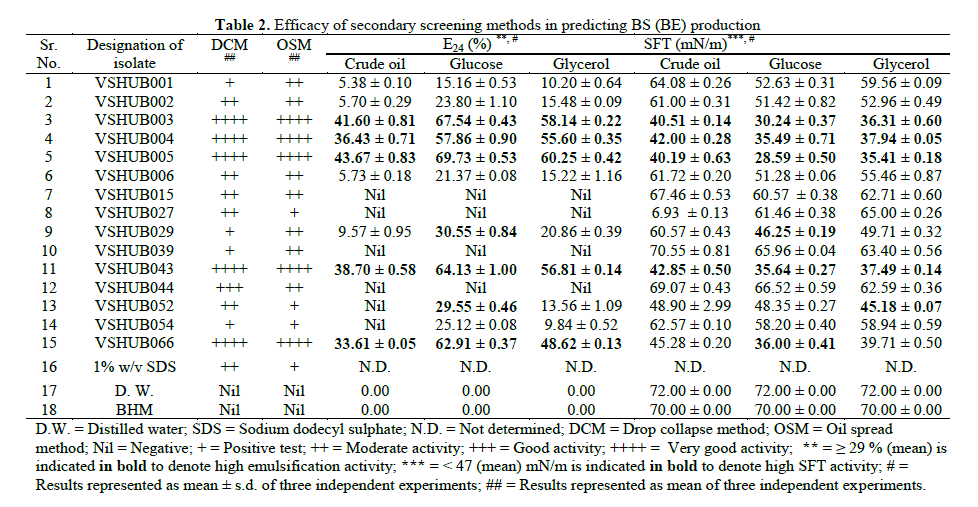
| 3.2 Secondary Screening: 15 potential BS (BE) producing isolates obtained from 69 isolates by primary screening methods were used for secondary screening. Jain et al. suggested the use of DCM as a sensitive and easy method to test for BS production. The method can also be made quantitative [19]. However in this study DCM was applied as a qualitative method to detect biosurfactant production (Table 2). Among 15 isolates 4 (26.66%), 5 (33.33%), 1 (6.66) and 5 (33.33%) isolates showed positive test, moderate activity, good activity and very good activity, respectively for DCM. The area of displacement of hydrophobic substance by a surfactant-containing solution is directly proportional to the concentration of the BS present in it [20]. We tested whether OSM could be used to detect BS production by the fifteen isolates obtained by primary screening methods. Among 15 isolates 3 (20.00%), 7 (46.66%) and 5 (33.33%) isolates showed positive test, moderate activity and very good activity, respectively for OSM. OSM results were found in corroboration with DCM results. Most of isolates found with positive DCM results were positive for OSM also (Table 2). These results confirmed the presence (for isolates with positive results) or absence (for isolates negative results) of BS. Reliability of results obtained in DCM and OSM in this study was similar to the results reported by Thavasi et al. [40]. DCM and OSM were reported as an indicative of the surface and wetting activities [18]. The advantage of DCM and OSM is very easy and less sample volume (10 to 20μl) is required to check the drop collapse or oil spread, which helps in repeating the measurements [40]. Also it can be performed without an aid of specialized instrument. Sometimes low sensitivity is observed in case of DCM as significant concentration of surface active compounds is not present in order to cause a collapse of the aqueous drops on the oil or glass surfaces [6]. From earlier reports and results of this paper DCM and OSM are recommended as a reliable, rapid, simple and consistent analytical method (s) for accurate measurement for BS production [18-20,40]. For E24 and SFT measurement studies the fifteen isolates selected were grown on BHM supplemented with crude oil (1% v/v), glucose (1% w/v) and glycerol (1% w/v). E24 is reported as an indirect method used to screen BS production [40]. Using crude oil, glucose and glycerol as carbon source 9 (60%), 11 (73.33%) and 11 (73.33%) isolates, showed positive results for E24, for the respective substrates (Table 2). Whereas quiet different results were noted for SFT measurement, i.e. all 15 (100%) isolates showed more or less surface activity when SFT was measured. When grown on crude oil, glucose and glycerol as sole source of carbon and energy in BHM 4 (26.66%), 6 (40%) and 5 (33.33%), isolates showed SFT ≤ 47 mN/m. E24 ≥ 29% and SFT ≤ 47 mN/m was considered for selection of the isolates for further screening by interfacial tension measurement (Table 2). OSM, DCM and SFT results were in corroboration with each other. E24 is considered for BE production, and SFT reduction was considered for BS production. In present study some isolates showing good and notable results in SFT measurement but no notable results were obtained with E24, suggested that all BS producers are BE producer but not vice versa. Reports are available indicating that always correlation between surface activity and emulsification capacity cannot be found i.e. E24 method gives just an indication on the presence of BS [6]. Markande et al. reported BE as a subclass of high molecular weight surfactant [9]. The most useful advantage of SFT is the accuracy of the method [6]. However SFT measurement requires specialized equipment which is costly and not necessarily available in all microbiological laboratories. Other disadvantages are that measurements of different samples cannot be performed simultaneously, and at least 15 - 20 ml of sample is required for analysis [6,19]. |
| 3.3 Tertiary screening: By using secondary screening methods (DCM, OSM, E24 and SFT measurement) seven isolates (VSHUB003, VSHUB004, VSHUB005, VSHUB029, VSHUB043, VSHUB052 and VSHUB066) were selected for their BS production by tertiary screening method i.e. IFT measurement. All these isolates were grown on BHM supplemented with crude oil (1% v/v/), glucose (1% w/v) and glycerol (1% w/v). The results for IFT measurement are shown in Fig. 1. Along with all isolates IFT was measured for distilled water (D.W.) i.e. negative control. IFT measurement for BHM was also checked. No IFT reduction was noted in case of D.W. and BHM. From these seven isolates only 1 (14.28%) isolate i.e. VSHUB005 showed IFT reduction ≥ 80% when grown on all three carbon sources, i.e. crude oil, glucose and glycerol. This is the first report where IFT measurement is included as one of the method for BS producer screening. From the above studies including E24, SFT and IFT measurement amongst the carbon sources used to grow bacterial isolates glucose (1% w/v) was proved to be better one for BS production. Maximum E24 (69.73 ± 0.53%), SFT measurement (28.59 ± 0.50 mN/m) and IFT reduction (90.46 ± 0.08%) was noted for the isolate VSHUB005 when grown using BHM supplemented with 1% w/v glucose as C-source and was selected as potent BS (BE) producing HUB isolate. |
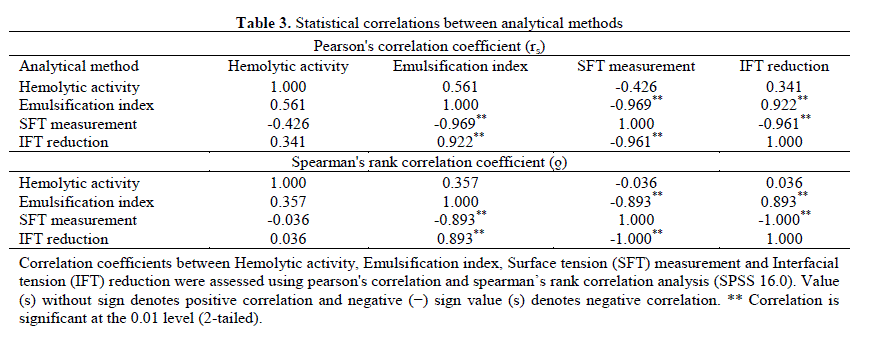
| 3.4 Statistical analysis: The results of statistical correlations between different analytical methods viz. HA, E24, SFT measurement and IFT reduction are represented in Table 3. There was a very strong, negative correlation between the SFT measurement and IFT reduction (rs = - 0.961, ÃÂ = -1.000); SFT measurement and E24 (rs = - 0.969, ÃÂ = -0.893); a strong positive correlation between the E24 and IFT reduction (rs = 0.922, ÃÂ = 0.893) was observed, which suggests that the SFT measurement and IFT reduction provides better results than E24. Between HA and IFT measurement weak negative Pearson’s correlation coefficient (rs = - 0.426) and very week positive Spearman’s correlation coefficient (ÃÂ = 0.036) was noted, which indicates that HA can be considered as primary screening method to detect BS production. Youssef et al. and Afshar et al. reported very weak correlation between SFT and HA method with the reported value ÃÂ = -0.15 and rs = -0.007, ÃÂ = 0.036, respectively [32,33]. However weak correlation between OSM and HA (ÃÂ = 0.497); DCM and HA (ÃÂ = 0.528) was reported by Youssef et al. [32]. Based on statistical comparison and other results in this study it can be concluded that there is a good consistency between DCM, OSM, SFT measurement and IFT measurement. DCM and OSM followed by E24, SFT and IFT assessment (s) were reliable for screening purpose. |
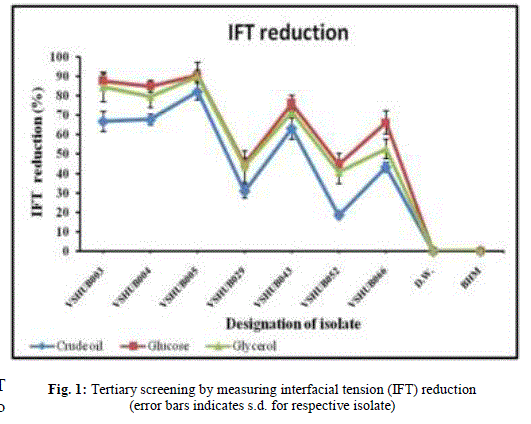 |
 |
 |
 |
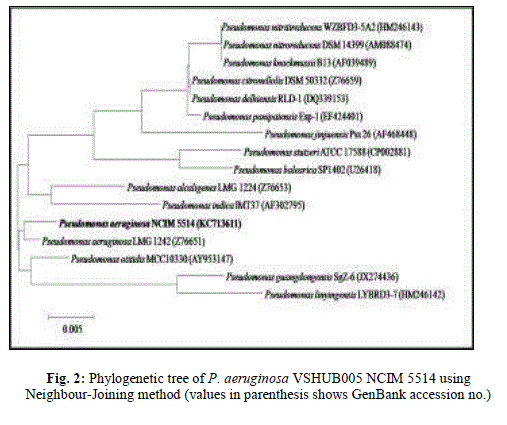
| 3.5 Characterization and identification of BS (BE) producing isolate: VSHUB005 is a gram-negative short rod. It produced medium, irregular, flat, smooth, translucent colonies with diffusible fluorescent yellow-green pigment on N. agar medium. This isolate was identified as Pseudomonas aeruginosa at 99% probability with excellent confidence level by VITEK®2 Compact system. VSHUB005 was identified as P. aeruginosa using 16S rDNA gene sequencing, and the sequence has been submitted to GenBank database under accession number KC71361, whereas its NCIM ID is 5514. Phylogenetic tree showing evolutionary relationship of this P. aeruginosa VSHUB005 with selected fifteen Pseudomonas sp. is shown in Fig. 2. Several authors have researched, reviewed and suggested the use of BS (BE) producing bacterial isolates of different genera in field of bioremediation and enhanced oil recovery. Pseudomonas is one of the most reported in this category [3,40,41,42]. Wang et al. reported 785.4 ± 23.5 and 501.4 ± 17.2 mg/l rhamnolipid production in 4 days (30°C, 250 rpm) by P. aeruginosa PEER02 and P. aeruginosa PAO1, respectively using mineral salt medium supplemented with 2% w/v glucose and suggested the role of rhamnolipid BS in enhanced oil recovery [25]. |
 |
IV. CONCLUSION |
| Seven screening methods namely (HA, CTAB agar plate, DCM, OSM, E24, SFT measurement and IFT reduction methods) were employed for BS (BE) and assessed for their suitability for this purpose. P. aeruginosa NCIM 5514 (GeneBank accession number KC713611) was selected as a potential BS (BE) producing isolate from 69 HUB isolates obtained from petroleum contaminated sites and used for screening purpose in this study. Glucose was a better carbon source than crude oil and glycerol for growth of VSHUB005. This is the first report to employ IFT measurement as one of the screening methods for BS (BE) producers. Strong negative correlation between SFT measurement and IFT reduction justifies the inclusion of IFT measurement as a screening method for BS (BE) producers. The sequence of protocols to be followed as presented here provides a rapid and effective way for the selection of bacterial isolates for BS (BE) production. P. aeruginosa NCIM 5514 could be used for field trials in bioremediation and enhanced oil recovery after optimizing growth conditions for culture growth and BS (BE) production. |
ACKNOWLEDGEMENT |
| We express our gratitude to Gujarat National Law University (GNLU), Gandhinagar, Gujarat for granting permission to Ms. Sunita to perform research work. We are grateful to Gujarat Ecology Commission (GEC), Gandhinagar and Institute of Reservoir Studies (IRS), ONGC, Ahmedabad. Laboratory facility provided at M. G. Science Institute (DBT, “Star College Scheme Grants”) and Pandit Deendayal Petroleum University (PDPU) is highly acknowledged. We are thankful to Dr. M. B. Thaker for his support in statistical analysis. |
References |
|