ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
ShanmugaSigamani1, PR. Thangavelu2, K N Srinivasan3, M.Selvam4
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Anodizing is an electrolytic oxidation process. Good amount of work has been carried out in various electrolytes using direct current (DC). Anodizing may be regarded as the artificial thickening of the thin (1–5 nm) native oxide film that is always present on aluminum exposed to the atmosphere. In this investigation, a porous oxide coating has been formed on aluminum by using alternating current (AC) anodizing under a current density range of 1–5 A/dm2 and temperature of 15–350C in sulfuric acid electrolyte containing sodium sulfate. There is an increasing trend on the coating ratio up to 3 A/dm2. Anodizing time has some effect both on coating ratio and on anodic film thickness. Similarly, temperature of the electrolyte greatly affects both thickness and coating ratio. Increasing electrolyte temperature decreases both thickness and coating ratio. Addition of sodium lauryl sulfate (SLS) to the AC anodizing electrolyte improves the coating ratio even up to the current density of 5 A/dm2. When compared with plain bath addition of gelatin along with SLS improves the finish of the anodic coating as well as coating ratio. In AC anodizing, gelatin slightly improves the finish of the anodic coating; moreover, due to its inhibiting action on the surface gelatin reduces the dissolution of anodic coating thereby slightly improves both thickness and coating ratio compared with plain bath.
Keywords |
| ac anodizing; anodic coating ratio; aluminum oxide; SLS and gelatin additive to aluminum anodizing |
I. INTRODUCTION |
| Anodizing is an electrolytic oxidation process in which an aluminium component is made anodic in a cell containing an aqueous, acidic electrolyte and a metal cathode. The widely used anodizing electrolyte is sulphuric acid1- 7. When electric current is passed through the cell, the aluminium surface is converted to an adherent aluminium oxide coating which is integral with the aluminium substrate. Anodising may be regarded as the artificial thickening of the thin (1 to 5 nm) native oxide film that is always present on aluminium exposed to the atmosphere. During anodising, hydroxyl ions from the electrolyte are driven to the aluminium surface where they penetrate the existing oxide film and combined with aluminium leads to oxide coating near metal/oxide interface. |
| A non-porous barrier oxide forms initially and persists as an advancing front into the metal during the anodising process. Porous oxide coating soon develops above the barrier zone due to the dissolving action of the acidic electrolyte. The porous structure permits continued growth in thickness of the coating until equilibrium is established between formation and dissolution of coating. |
| 50 Hz AC along with a dimmer stat is normally being used for anodizing where both electrodes get anodized during the process. It has got considerable commercial use but it has not been successfully used for decorative, architectural and continuous anodizing. This is because of some difficulties encountered in AC anodizing. When anodizing aluminium in sulphuric acid electrolyte is carried out, in addition to hydrolysis of water, sulphuric acid is get reduced and sulphur and its compounds get deposited inside the pores of the oxide film resulting in voltage raise followed by streaking and petting. |
| Sacchi and Paoline [8] compared AC anodizing in sulphuric acid with DC over a relatively narrow range of conditions (20% w/v H2SO4, 16-250C, 100-250A/m2). W.E. Crooke studied the effect of temperature, current density and time using AC in either 15 or 18% w/v sulphuric acid [9]. AC sulphuric acid has been modified by the use of addition agents which shifts the basic mechanism of electrolysis back in favour of the electrolysis of water rather than the reduction of sulphuric acid [10-13]. Alkali metal sulphate, nitrate, borate, citrate, tartrate and oxalate (2 –5% w/v) are used and the parameters such as concentration of addition agent, anodizing electrolyte and operating conditions like current density and temperature have been studied [14]. |
| Modifiers are reported by D.R.Gabe [15] in the AC anodizing of aluminium to increase film thickness and to improve the appearance of the finish. These modifiers are generally oxidants but transition metal ions in a high valency state are also found to be particularly effective. The oxidant can depolarise the hydrogen evolution reaction. The formation of anodic oxide in AC is essentially similar to DC but the balance between oxide formation at the inner surface and oxide dissolution at the outer surface. |
| In the present work a detailed studies have been carried out on AC anodizing of aluminium in 10% sulfuric acid containing 3% sodium sulfate at various temperatures and current densities with and without organic additives on the coating ratio, thickness, hardness and topography. |
II. EXPERIMENTAL |
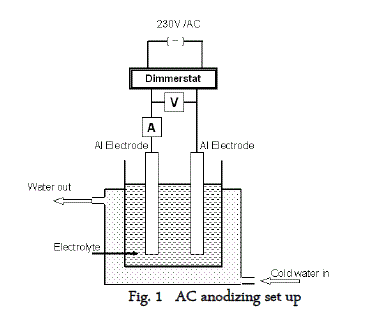
| 2S aluminium panels of 1cm x 7cm were subjected to alkaline cleaning in 5% NaOH solution for 5mts followed by desmutting in 25% HNO3 for 2 minutes, washed, rinsed, dried and weighed. Cleaned and weighed (W1) aluminium panels were anodized in 10% H2SO4 solution containing 3% sodium sulfate at different temperatures, times and current densities with and without additives. For AC anodizing 5A dimmerstat was used along with two multimeters, one at the series connection to measure the current and one at the parallel connection to measure the voltage across the electrodes. The AC anodisation block diagram is given in figure 1. |
| Double walled plastic container was used as the anodizing cell. Cold water was circulated through the double wall using a fractional horse power pump so as to maintain the electrolyte temperature at the required level. |
| Thus anodized aluminium was weighed (W2) and subjected to stripping in a solution containing chromium trioxide 20 g/l and phosphoric acid 35 ml/l washed, rinsed, dried and weighed (W3). From these weights the coating ratio was calculated as follows |
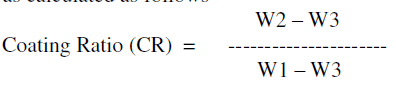 |
| W2 – W3 = Weight of the oxide coating |
| W1 – W3 Total weight of Al reacting |
 |
| Thickness of the anodic coating was calculated as follows from the weight of anodic coating, surface area and density of anodic film. |
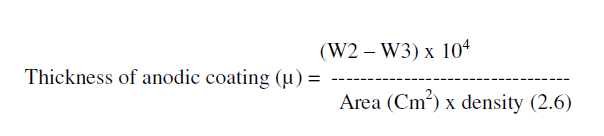 |
III. RESULTS AND DISCUSSION |
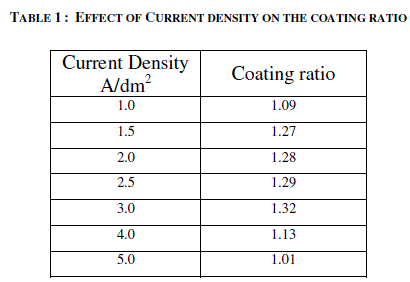
| 3.1 EFFECT OF CURRENT DENSITY ON THE THICKNESS OF THE ANODIC COATING AND COATING RATIO |
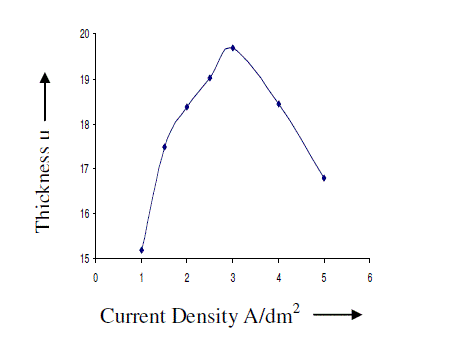
| AC anodizing was carried out at various current densities (1-10 A/dm2) with an almost constant charge by varying the anodisation time. The temperature of the electrolyte was kept at 200C. The effect of current density on the thickness of the anodic coating is recorded in figure 2. Effect of Current density on the coating ratio is presented in table 1. It is seen from the figure 2 and table 1 that there is an increasing trend in both thickness and coating ratio up to a current density of 3A/dm2. It is well known that during anodisation, apart from the formation of anodic coating there is also dissolution of the anode. Above 3A/dm2 the dissolution rate is higher comparing with dissolution occurs at lower current densities. High CD in turn increases the local temperature7 at the surface of the anode facilitates the dissolution of oxide coating leads to low coating ratio. |
| Based on this, for further studies, 2 A/dm2 was chosen as the optimum current density. |
 |
 |
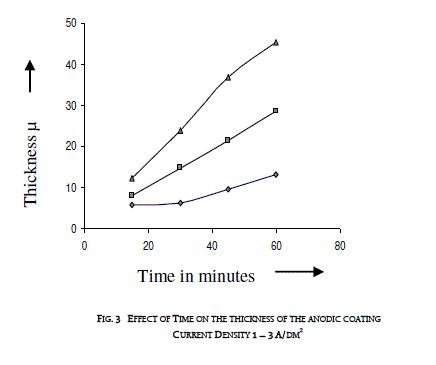
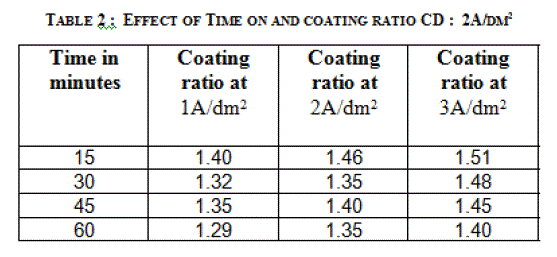
| 3.2 EFFECT OF TIME ON THE THICKNESS OF THE ANODIC COATING AND COATING RATIO |
| Anodization time was varied between 15 and 60 minutes by keeping a constant current density and temperature (200C). Effect of time on the thickness of the anodic coating is given in figure 3 and coating ratio in table 2. Increasing anodization time increases the coating thickness gradually at lower current density. There is a linear increase in coating thickness at 2A/dm2. However, there is a reduction in coating ratio at higher duration irrespective of the current density. From this experiment anodization time of 45 minutes was chosen as the optimum value. |
 |
 |
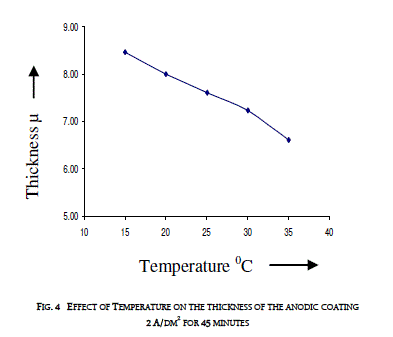
| 3.3 EFFECT OF TEMPERATURE ON THE THICKNESS OF THE ANODIC COATING AND COATING RATIO |
| Temperature of the electrolyte was varied between 15 and 350C by keeping the other variables at constant values i.e. 2 A/dm2 for 45 minutes. Effect of temperature on the thickness of the anodic coating is presented in figure 4 and coating ratio is given in table 3. Effect of Temperature on coating ratio is presented in Table 3. |
 |
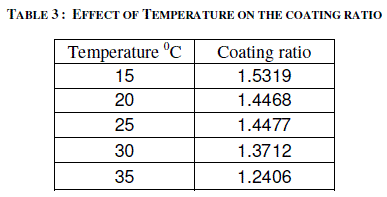 |
| It is seen from the figure and table that there is always a decrease in trend in both coating thickness and coating ratio with increase in bath temperature. Temperature of the electrolyte greatly affects both thickness and coating ratio. This clearly indicates that higher temperature favors more dissolution than the low temperatures. Increasing temperature also decreases the coating ratio. Though temperature of the electrolyte also influences the coating ratio 200C was chosen as the optimum temperature for this study. |
| 3.4 EFFECT OF ORGANIC ADDITIVES |
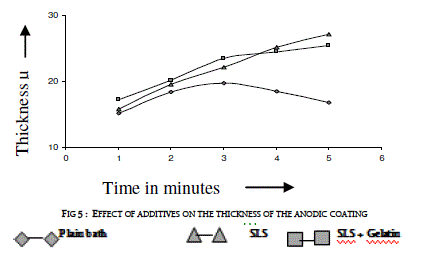
| Sodium lauryl sulfate (SLS) of 0.2 g/l was added to the electrolyte as a wetting agent. In plating gelatin is added as a grain refinement additive. It was tried in AC anodizing by the addition of 0.5 g/l to the electrolyte. Effect of current density with and without additives on the thickness of the anodic coating and coating ratio are presented in figure 5 and in table 4 respectively. |
 |
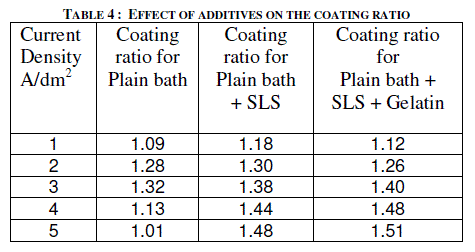 |
| Addition of both SLS and gelatin slightly improves the coating ratio. That means it slightly reduces the dissolution at higher current densities above 3 A/dm2. With out these additives there is a decrease in coating thickness as well as coating ratio beyond 3 A/dm2. In general, gelatin in electro plating baths gets adsorbed at the electrode and does refining the crystal growth by which improved finish could be formed. In AC anodizing gelatin slightly improves the finish of the anodic coating. Further due to its inhibiting action on the surface it reduces the dissolution of anodic coating thereby slightly improves both thickness and coating ratio compared with the plain bath. |
| 3.5 SEM STUDIES |
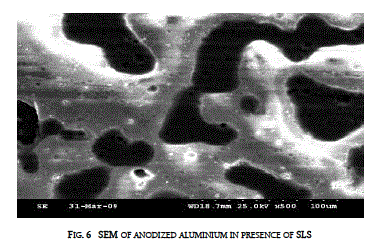
| The surface topography of the anodized aluminium was found out using Scanning Electron Microscope (SEM - JEOL-Japan-JSM-840A) in presence of SLS and along with gelatin is presented in figures 6 and 7. |
 |
 |
| Addition of both SLS and gelatin modified the topography of the anodized aluminium. Gelatin along with SLS improves the compactness of the anodic coating |
| 3.6 HARDNESS OF AC ANODIZED ALUMINIUM |
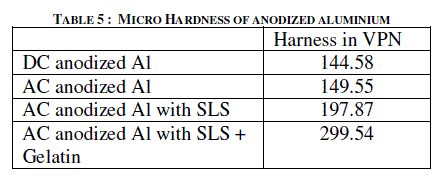
| Micro hardness of the anodized aluminium was found out using Everone Micro hardness Tester MH6, USA. Micro hardness values in Vickers Pyramid Number (VPN) for AC anodized with and without organic additive are given in Table 5. Table 5 shows that there is not much change in hardness values of DC and AC anodized samples. Where as addition of SLS enhances the value from 149.55 to 197.87. Further, addition of gelatin along with SLS greatly increases the harness value of 299.54. The increase in harness value is mainly due to the compactness observed in the anodic coating produced using AC in presence of organic additive in the sulfuric acid electrolyte containing sodium sulfate. |
 |
IV . CONCLUSION |
| AC anodizing in 10% sulfuric acid containing 3% sodium sulfate and organic additives SLS and gelatin produces improved coating ratio as well as its finish. Though SLS additive produces improved coating ratio the anodic coating is not as compact as with gelatin. Due to compactness of the anodic coating, produced when both SLS and gelatin are present in the electrolyte, it shows an increased hardness value. Further, sulfuric acid electrolyte containing sodium sulfate, SLS and gelatin could be operated at higher current densities >3 A.dm-2 in AC anodizing. |
References |
|