e-ISSN: 2320-0812
e-ISSN: 2320-0812
Nagaraju PT, K Venu Gopal, Murali Krishna NV*, Bhargavi V, and Srinivasulu N
Department of Pharmaceutical Analysis, Nirmala College of Pharmacy, Kadapa - 516002, Andhra Pradesh, India.
Received: 04 August 2014 Accepted: 22 September 2014
Visit for more related articles at Research & Reviews: Journal of Pharmaceutical Analysis
A simple, precise and economical second order derivative method has been developed for the estimation of Raloxifene in bulk and pharmaceutical formulations. In this method Raloxifene showed sharp peak at 288 nm when n= 1 and linearity was measured at 288 nm. It obeyed Beer’s law in the concentration range of 2-12 mcg/ml. The LOD and LOQ were found to be 0.32 mcg/ml and 0.98 mcg/ml respectively. A recovery of Raloxifene in tablet formulation was observed in the range of 99.38-100.60%. The proposed method is precise, accurate and reproducible and can be extended to the analysis of Raloxifene in bulk and pharmaceutical formulations.
Raloxifene, Spectrophotometric, Method validation.
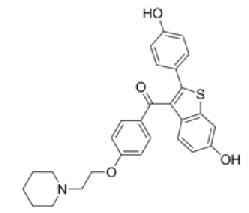
Raloxifene [10,11] is a Antihypocalcemic agent. Chemical name of Raloxifene is [6-hydroxy-2-(4-hydroxyphenyl) - benzothiophen-3-yl]-[4-[2-(1-piperidyl)ethoxy]phenyl] -methanone. It has a molecular formula of C28H27FNO4Sand a molecular weight of 478.583 g/mol.
Literature survey reveals that several analytical methods have been reported for the estimation of Raloxifene by UV[1,2,3,4,5], RP-HPLC [6,7],UPLC-MS [8] and LC-MS [9] methods. Apart from above one spectroscopic method such as UV/Vis, difference spectrophotometric method, RP-HPLC etc., were reported for this compound.
UV spectrophotometric method was reported for the quantitative determination of Raloxifene in pharmaceutical dosage forms. The developed method was simple, precise, specific and accurate and the statiscal analysis proved that method is reproducible and selective for the analysis of Raloxifene in bulk drug and tablet formulations.
A Elico-210 UV/VIS spectrophotometer was used with 1 cm matched quartz cell. All the chemicals used were of analytical grade. Methanol was procured from Loba Chem. Ltd., Mumbai. An analytically pure sample of Raloxifene was obtained from Hetero Drugs Limited as a gift sample.
The standard Raloxifene (100 mg) was weighed accurately and transferred to volumetric flask (100 ml). It was dissolved properly and diluted up to the mark with Methanol to obtain final concentration of 1000 mcg/ml and the resulting solution was used as working standard solution.
For the estimation of Raloxifene in tablets formulations by this method. 5 branded tablets were weighed and triturate to fine powder. Tablet powder equivalent to 10 mg of Raloxifene was weighed and transfer into 100 ml volumetric flask than dissolved with Methanol and further diluted with Methanol. It was kept for ultrasonication for 30 min; this was filtered through Whatman filter paper No. 41 and then final dilution was made with Methanol to get the final stock solution of 100 mcg/ml. From this stock solution, various dilutions of the sample solution were prepared and analysed.
The spectra showed sharp peak at 288 nm when n=1 and linearity was measured at 288 nm (Fig 1). The absorbance difference at n=1 (dA/d?) is calculated which was directly proportional to the concentration of the standard solution. The standard drug solution was diluted so as to get the final concentration in the range of 2-12 mcg/ml and scanned in the spectra. The calibration curve of dA/d? against concentration of the drug showed linearity. Similarly absorbance of sample solution was measured and amount of Raloxifene was determined from standard calibration curve.
As the drug Raloxifene showed a broad spectrum, the spectroscopy method applied has the advantage that it locate the hidden peak in the normal spectrum, when the spectrum is not sharp and it also eliminate the interference caused by the excipients and the degradation products present, if any, in the formulation.
The spectra showed sharp peak at 288 nm when n=1 and linearity was measured at 288 nm. The polynomial regression data for the calibration plots showed good linear relationship in the concentration range of 2-12 mcg/ml with r2= 0.999 and given in Table 1.
From stock solution (1000mg/ml) take 10ml and make up the dilution to 10ml with Methanol. The precision of the analytical method was studied by analysis of multiple sampling of homogeneous sample. The precision results were expressed as standard deviation or relative standard deviation.
b% RSD of the six replicate injections should not more than 2.0%.
Recovery studies were carried out at three different levels i.e. 80%, 100% and 120% by adding the pure drug to the previously analysed tablet powder sample and shown in Table 4. The percentage recovery value indicates non interference of the excipients used in formulation.
The robustness of an analytical procedure is a measure of its capacity to remain unaffected by small, but deliberate variations in method parameters and provides an indication of its reliability during normal usage.
The solution was prepared and observed in replicate for three times with (±2) wavelength i.e. 286nm and 292nm respectively.
Ruggedness is a measure of the reproducibility of a test result under normal, expected operating condition from instrument to instrument and from analyst to analyst.
% RSD should not more than 2.0%.
A spectrophotometric method for quantifying Ralista-60mg in formulation samples has been developed and validated. The proposed method is precise, accurate and reproducible and can be extended to the analysis of Raloxifene in bulk and tablet formulations.
We would like thank to Hetero Drugs Limited, Jadacherla for providing reference sample of Raloxifene respectively to facilitate this work and also to the Principal Dr K. Venu Gopal, Nirmala College of Pharmacy, Kadapa for providing facilities.