ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Sara Shaker1, Shirzad Zafarian2, CH. Shilpa Chakra3,K.Venkateswara Rao4
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Environmental pollution such as dyes has been excessively released into the environment and has created a major global concern. Congo Red is a benzidine-based anionic diazo dye with two azo groups. It is toxic to many organisms and is a suspected carcinogen and mutagen. The presence of Congo Red (CR) in water even at very low concentration is highly visible and undesirable. Present work is focused on synthesis of magnetite nanoparticles which showed a high adsorption capacity of Congo Red and is useful in removal of CR from wastewater. Magnetite (Fe3O4) nanoparticles have been successfully synthesized by Sol–Gel method by using Ferric nitrate (Fe (NO3)3.9H2O) and Ethylene glycol (C2H6O2) as precursors in different annealing temperatures. The obtained nanoparticles have been characterized by X-Ray diffraction (XRD), Scanning Electron Microscope (SEM), X-ray energy dispersive spectrometer (EDS) and Particle Size Analyzer. XRD measurements indicate that the obtained nanoparticles are single phase and the particle size increased by increasing the temperature.
Keywords |
| Sol-Gel Method, Magnetite Nanoparticles, water treatment, Congo Red. |
INTRODUCTION |
| Magnetite (Fe3O4) is a common magnetic Iron Oxide and it has a cubic inverse spinel structure with oxygen forming a FCC closed packing and Fe cationsoccupy the interstitial tetrahedral sites and octahedral sites [1]. The synthesis of magnetite nanoparticles has been intensively developed not only for its great fundamental scientific interest but also for many technological applications in biology, such as extraction of genomic DNA [2], ultrahigh density magnetic storage media[3], medical applications (such as targeted drug delivery, labelling, separation) [4-6]. Various methods have been developed to synthesize nanosized magnetite particles such as coprecipitation or precipitation, Sol–Gel method, Emulsions Technique, Mechanochemical Processing, Hydrothermal Preparation and DC Thermal Arc-Plasma Method [7]. Among these methods for metal oxides, sol–gel process offers several advantages compared to other methods, including good homogeneity, low cost, and high purity. Recently, sol–gel method has been developed for preparation of magnetite nanoparticles using metallo-organic precursors [8]. In this article, magnetite nanoparticles are successfully synthesized via sol–gel method combined with annealing using inexpensive, nontoxic ferric nitrate and ethylene glycol as starting materials. Dyes are used from different industries such as paper and plastics, leather, food, cosmetics, textiles, etc. to color the products. The presence of these dyes in water even at very low concentration is highly visible and undesirable [9]. Congo red [1-naphthalene sulfonic acid, 3,30-(4,40-biphenylenebis (azo)) bis (4-amino-)disodium salt, CR] is a benzidine-based anionic disazo dye, i.e. a dye with two azo groups. It is toxic to many organisms and is a suspected carcinogen and mutagen [10]. Various adsorbents have been tested and used for the removal of dyes from polluted water [11]. Magnetite nanoparticles were employed for removal of CR and used as an effective adsorbent in the wastewater treatment. The technique was found to be very useful and cost-effective for a better removal of dye. |
II. EXPERIMENTAL DETAILS |
| A. Materials |
| Ferric Nitrate (Fe (NO3)3.9H2O) and ethylene glycol (C2H6O2) of analytical grade were used to prepare Magnetite Nanoparticles and they were obtained from Finar chemicalscorporation. The reagents were used without further purification. |
| B. Synthesis of Magnetite Nanoparticles |
| The procedure of synthesizing magnetite nanoparticles described as follows: Ferric Nitrate and ethylene glycol are dissolved in proper ratios and was stirred for 2 h at 400C.Then, the prepared sol was heated to 800C obtain brown gel. The gel was aged at room temperature for about 1 h and then the xerogel was annealed at 200, 300 and 4000C in furnace under air atmosphere. Finally, magnetite nanoparticles in different sizes were synthesized, as shown in Fig.1. |
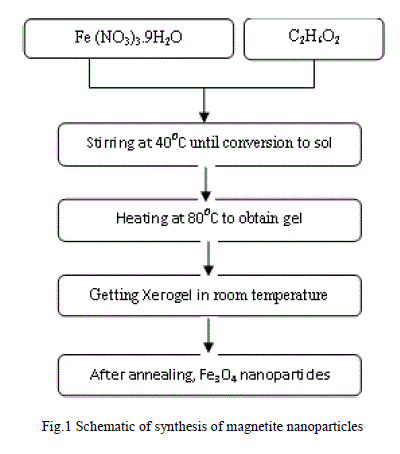 |
| C. Characterization The obtained d nanoparticles were characterized by X-Ray Diffraction (XRD) (XRD, Bruker D8 Advance) with Cu Kα radiation (λ = 0.15418 nm). The surface topography, and composition analysis of magnetite nanoparticles were obtained by using a Scanning Electron Microscopy (SEM)(S-3400) equipped with an X-ray Energy Dispersive Spectrometer (EDS) and the particle size were obtained by Particle Size Analyzer. |
III. RESULTS |
| A. XRD The diffraction peaks at 2θ = 300, 350, 430, 570 and 620can be assigned to (2 2 0), (3 1 1), (4 0 0), (5 1 1) and (4 4 0) planes of Fe3O4 (PCPDF#750033) in 2000C and 3000C. At 4000C there are more peaks at 260, 330 and 500 which can be assigned to (2 1 1), (3 1 0), (4 2 1) and compared to (PCPDF#391346), it is indicated that these peaks are related to γ- Fe2O3. The crystallite size of the nanoparticles is calculated by Scherrer formula: |
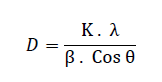 |
| Where K is a dimensionless shape factor, λ is the X-ray wavelength, β is the line broadening at half the maximum intensity (FWHM) and θ is the Bragg angle. The mean crystallite size of the Fe3O4 nanoparticles synthesized at 2000C, 3000C and 4000C are 28.7 nm, 30.5 nm and 34.9 nm respectively. It shows that with raising annealing temperature the sizes of Fe3O4 nanoparticles increase, as shown in Fig.2. |
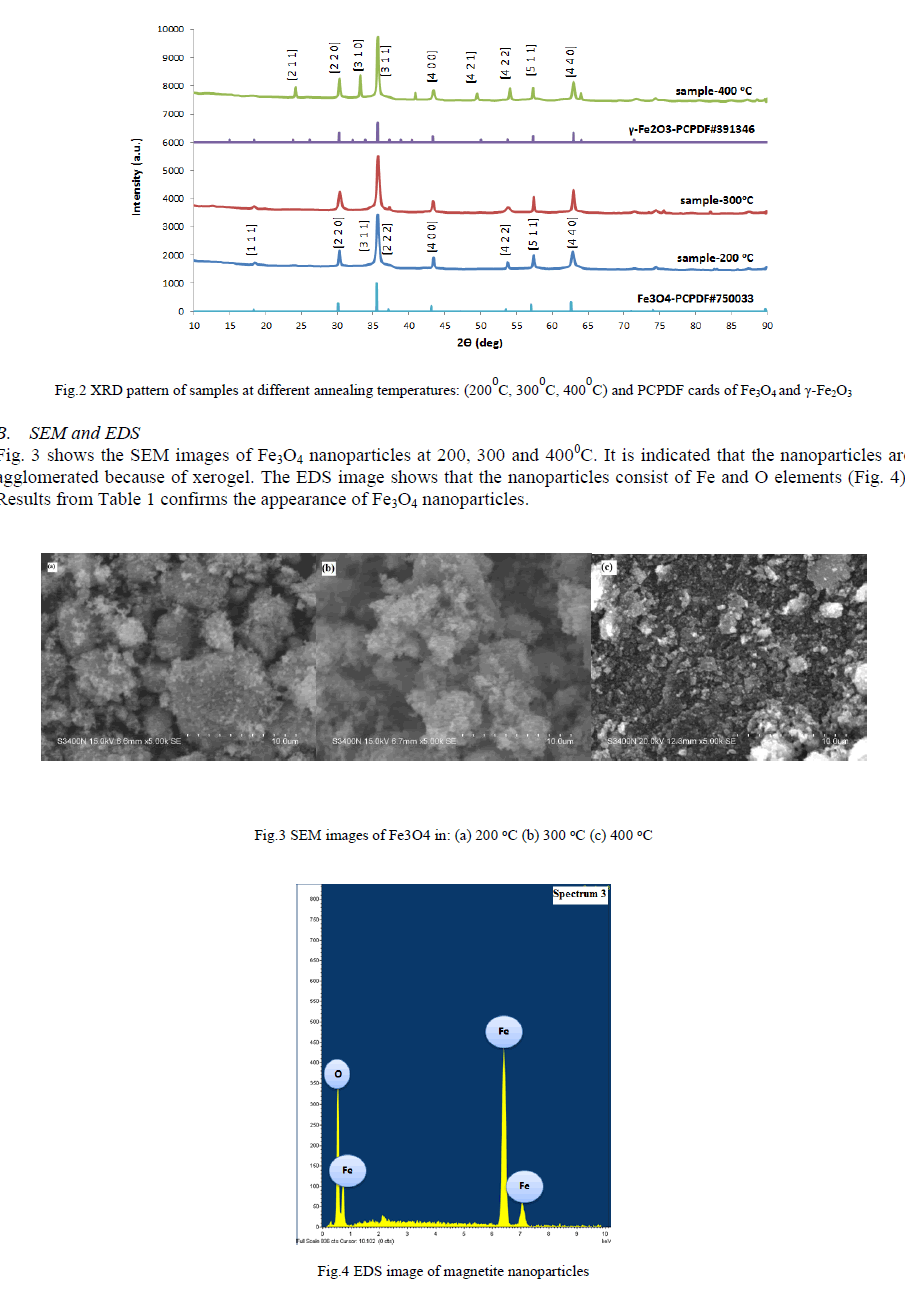 |
 |
IV. DISCUSSION |
| The synthesized Fe3O4 nanoparticles can be transformed easily into γ-Fe2O3, α-Fe2O3 or α-Fe by treating at different temperatures and atmospheres. It is known that Fe3O4 nanoparticles can be oxidized to γ -Fe2O3, which can be further transformed into α-Fe2O3 at higher temperature [12]. The result indicates that the oxidation of Fe3O4 in air at 4000C leads to γ-Fe2O3 and the annealing temperature under air must be in the range between 200 – 3500C. According to [7], the transformation of Fe3O4 to α-Fe2O3 can be observed at 5000C in air. Due to an organic reagent (ethylene glycol) as starting material and the closed system for annealing reaction, Fe3O4 nanoparticles might absorb some reductive materials of organic residual materials on their surfaces and Fe3O4 nanoparticles are reduced into α -Fe at 9000C by absorbing organic residual materials on their surfaces. |
V. CONCLUSION |
| Fe3O4 nanoparticles were prepared by sol–gel method combined with annealing temperature of 200, 300 and 400 0C. The characterization results show that the size of Fe3O4 nanoparticles can change by varying the annealing temperature. The Sol-Gel method offers several advantages for preparation of Fe3O4 nanoparticles. First, the synthetic process is economical and environmentally friendly, because it involves inexpensive and less toxic iron salts. Second, sizecontrolled Fe3O4 nanoparticles are produced by different annealing temperatures. Among the kinds of adsorbents, particularly magnetic iron oxides such as magnetite (Fe3O4) have been investigated intensively for environmental and Bio-Applications. Fe3O4 nanoparticles show convenient magnetic properties, low toxicity and price, high surface area to volume ratio, which are associated to their ability for surface chemical modification and show enhanced capacity for removing pollution such as dyes in water treatment. One of these dyes is Congo red which is banned in many countries because of health concerns. |
ACKNOWLEDGMENT |
| I would like to thank Dr. KhashayarBadii the Assistant Professor of Institute for Colour Science and Technology (ICST) in Iran for guiding me in this project. |
References |
|