e-ISSN: 2319-9849
e-ISSN: 2319-9849
M Sri Lakshmi1, R Ramesh Raju2, C Rambabu2, GV Rama Rao3, K Narendra4*
1Department of Chemistry, V.R. Siddhartha Engineering College, Vijayawada-07, India
2Department of Chemistry, Acharya Nagarjuna University, Guntur, India
3Department of Physics, Dharma Apparao College, Nuzvid, India
4Department of Physics, V.R. Siddhartha Engineering College, Vijayawada-07, India
Received date: 23/12/2012; Revised date: 03/02/2013; Accepted date: 07/02/2013
Visit for more related articles at Research & Reviews: Journal of Chemistry
Ultrasonic velocity, density and viscosity are measured in binary liquid mixtures of cyclohexanone with n-heptanol, n-octanol and iso-octanol at temperatures (303.15, 308.15, 313.15 and 318.15) K over the entire range of composition. By using these data, parameters such as adiabatic compressibility, β, molar volume, V are calculated and some excess parameters like excess adiabatic compressibility, βE, excess molar volume, VE, deviation in viscosity, Δη, and excess Gibb’s free energy of activation for viscous flow, ΔG*E are calculated. The calculated excess and deviation functions have been fitted to the Redlich-Kister polynomial equation. The observed negative and positive excess values or deviation parameters have been explained in the light of intermolecular interactions present in these binary liquid mixtures.
excess molar volume, deviation in viscosity, cyclohexanone, alcohols
Ultrasonic measurements are very useful in chemical and food processing, material testing, under water ranging and cleaning. Ultrasonic vibrations are commonly employed in mechanical machinery of materials [1], preparation of colloids or emulsions, the pregermination of seeds, for imaging of biological tissues [2], activation energy of metabolic process [3], formation and destruction of azeotropes in petrochemical industries[4] and in non-destructive testing (NDT). Study of thermodynamic properties of binary mixtures of varying composition and environment provides opportunities for continuous adjustment of observable properties and thus, yields an experimental background for optimizing the choice of solvent in manifold applications.
The extraction of benzene, toluene, ethylbenzene and xylenes from refinery products such as naphtha, kerosene and fuel jets is very important to the petrochemical industry. Because of this, the information about the physical properties of pure liquids and liquid mixtures containing aromatic and aliphatic compounds and their dependence with composition and temperature is very important basic data.
The study of the possible changes of thermodynamic properties of mixtures and their degrees of deviation from ideality has been found to be an excellent qualitative and quantitative way to obtain information about the molecular structure and intermolecular forces in liquid mixtures. This has given impetus to the theoretical and experimental investigations on excess thermodynamic properties of liquid mixtures.
Properties of liquid-liquid mixtures are thermodynamically very important as part of studies of thermodynamic, acoustic and transport aspects. The intermolecular forces of liquids in a mixture show a considerable effect on the physical and chemical properties[5-9]. Thermodynamic and hydrodynamic properties of cyclohexanone with 1-alkanols are important in the fundamental understanding of mixing processes and in many practical problems concerning chemical separation, heat transfer, mass transfer and fluid flow.
Formation of hydrogen bonding between the molecules of a liquid mixture is a common chemical effect that influences thermodynamic properties of solutions to a greater extent than any other specific and physical interactions[10,11]. Molecules containing hydrogen linked to an electro negative atom, exhibit a tendency to associate with each other and to interact with other molecules possessing accessible electronegative atoms. The extent of self – association and intermolecular bonding between unlike molecules vary with the composition of the mixture, for instance the dilution of a strong hydrogen bonds and gradual decrease in the concentration of aggregates. This would be attended by absorption of heat as well as expansion in volume. The magnitude of volume change can be determined by the structure breaking ability of the inert solvent. If the unlike molecules participate in hydrogen bonding, it would result in evolution of heat and contraction in volume. In mixtures, where both the aforesaid effect compete one another, the sign of excess function will depend on the predominant effect. Solvent structure determines the nature of interactions between like and unlike molecules of a liquid binary mixture. It also provides basic information used in evaluating the solute-solvent interactions.
Ultrasonic velocity, density and viscosity values are measured in binary liquid mixtures cyclohexanone with n-heptanol, n-octanol and iso-octanol at temperatures (303.15, 308.15, 313.15 and 318.15) K over the entire range of mole fraction. By using these data, the parameters like adiabatic compressibility, molar volume and some excess parameters like excess adiabatic compressibility, βE, excess molar volume VE, deviation in viscosity, Δη, and excess Gibb’s free energy of activation for viscous flow, ΔG*E are calculated. The calculated excess and deviation functions have been fitted to the Redlich-Kister polynomial equation. The observed negative and positive excess values or deviation parameters have been explained on the basis of the intermolecular interactions existing in these binary liquid mixtures.
Materials
The mass fractions of the liquids, alcohols (obtained from SRL Chemicals, Mumbai) are as follows: n-Heptanol (0.99), n-octanol (0.990) and iso-octanol (0.990) and that of the liquid, cyclohexanone (obtained from Merck, Bangalore) is 0.99. All the liquids obtained from the suppliers were further purified by standard procedure [12].
Method
To prepare the mixtures in the required proportions Job’s method of continuous variation was used and the mixtures were preserved in well-stoppered conical flasks. The flasks were left undisturbed to allow them to attain thermal equilibrium.
The mass measurements were performed on a Shimadzu AUY220, Japan, electronic balance with a precision of ± 0.1 mg was used for the mass measurements. Densities of pure liquids and their mixtures were determined by using a specific gravity bottle with an uncertainty ± 0.5%.
Ostwald’s viscometer calibrated using water and benzene has been used to measure viscosities at the desired temperature. The flow time has been measured after the attainment of bath temperature by each mixture. An electronic stopwatch with a precision of 0.01 s has been used to measure the flow time. For all pure compounds and mixtures, 5 to 7 measurements were performed and the average of these values was used in all the calculations. The uncertainty of viscosity was ± 10-3 m Pa.s.
A single crystal ultrasonic pulse echo interferometer (Model: F-80X Mittal enterprises, India), equipped with a high frequency generator and a measuring cell has been used for measuring ultrasonic velocities. These measurements were made at a fixed frequency of 3 MHz. By measuring the velocity in carbontetrachloride and benzene the equipment was calibrated. The results are in good agreement with the literature reports[13]. The error in velocity measurement is ± 0.5 %. The temperature was controlled through the water circulation around the liquid cell using thermostatically controlled constant temperature water bath with an uncertainty ± 0.01 K.
The experimental values of density (ρ), viscosity (η) and ultrasonic velocity (u) for all the mixtures over the entire range of composition and at T= (303.15, 308.15, 313.15 and 318.15) K are presented in Table 1.
From the experimental values of density, viscosity and ultrasonic velocity, adiabatic compressibility, molar volume were calculated using the equations,

where ρ is the density of the mixture and u is the ultrasonic velocity.

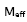 is given by
is given by 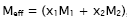 where M1 and M2 are the molar masses of pure components.
where M1 and M2 are the molar masses of pure components.
The strength of interaction between the component molecules of the binary mixtures is well reflected in the deviation of the excess functions from ideality. The excess properties such as excess adiabatic compressibility, βE, excess molar volume, VE, viscosity deviation, Δη, and excess Gibb’s free energy of activation for viscous flow, ΔG*E have been calculated using the standard equations [14]. The excess values of above parameters for each mixture have been fitted to the polynomial equation [15]
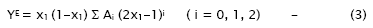
The values of the coefficients Ai were calculated by the method of least squares along with the standard deviation σ(YE). The coefficient is adjustable parameters for a better fit of the excess functions. The standard deviation values were obtained from
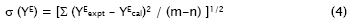
Where m is the number of experimental points, n is the number of parameters, Yexpt and Ycal are the experimental and calculated parameters, respectively.
The values of the Redlich–Kister polynomial coefficient Ai evaluated by the method of least squares along with standard deviation are given in Table 2.
The experimental values of ultrasonic velocity, density and viscosity at different mole fractions for different temperatures are listed in table 1. From these results, it is observed that the velocity increases and viscosity decreases with the increase in concentration of cyclohexanone. Both velocity and viscosity decrease with increase in temperature at any particular concentration.
The increase in mole fraction of cyclohexanone increases the net dispersive interactions and hence the velocity continuously increases as observed. In the case of alcohols, the methyl group becomes more non polar with increase in chain length. Further, the –OH group can dissolve preferably the polar component. So, in the lower mole fraction ranges, the added cyclohexanone has practically no interaction with the methyl or hydroxyl group of alcohols, thus sound velocity seems to decrease. Cyclohexanone being non polar, the predominant dispersive type interactions with temporary dipolar type are existed as a net result of intermolecular forces in all the systems, but more in iso-octanol system. These observations are in agreement with the general trends of the ultrasonic velocity variations in binary liquids [16-18].
Further, increase in temperature decreases the interaction due to thermal agitation, which is obvious from the decrease in ultrasonic velocity at higher temperatures. Similar observations were made by Syal et al [19] and Subramaniyam Naidu & Ravindra Prasad [20] in the investigation of temperature dependence of ultrasonic velocity in certain binary liquid mixtures. The ultrasonic velocity values decrease with increase of temperature due to breaking of hetero and homo molecular clusters at higher temperatures. A reduction in viscosity with increase in mole fraction of cyclohexanone suggests that the existing intermolecular interactions are weakening in magnitude. However, the increasing sound velocity with increasing mole fraction of cyclohexanone leads to a notion that the system is getting more and more compact. Figure 1 (a to c) – Figure 4 (a to c) show the variations of excess properties and deviations with the mole fraction of cyclohexanone. The results indicate that the excess adiabatic compressibility, βE, excess molar volume VE and excess Gibb’s free energy of activation for viscous flow, ΔG*E are positive in the entire composition range.
The variation of βE with the composition of mixture of cyclohexanone is presented in Figure 1(a to c). From the figures, it is observed that βE reaches a maximum at about 0.5 mole fraction for all the temperatures studied. Kiyohara and Benson [21] have suggested that βE is the resultant of several opposing effects. A strong molecular interaction through charge transfer, dipole induced dipole and dipole dipole interactions, interstitial accommodation and orientation ordering leading to a more compact structure makes βE negative and break up of the alcohol structures tends to makes βE positive. The βE values are positive for binary liquid mixtures of cyclohexanone with n-heptanol, n-octanol and iso-octanol. The positive values of βE for mixtures of cyclohexanone with n-heptanol, n-octanol and iso-octanol signify de-clustering of alcohols in the presence of cyclohexanone leading to positive βE values. The positive deviations in adiabatic compressibility increase with increase in temperature indicating the weakening of strength of interactions at higher temperature.
The variation of VE with the mole fraction of cyclohexanone for these systems at different temperatures is presented in Figure 2(a- c). It is seen that the VE values are positive for all the systems over the entire composition range at different temperatures. It is a well known fact that alcohols are self associated through hydrogen bonding. The mixing of cyclohexanone with alcohols is expected to induce changes in hydrogen bonding equilibrium and electrostatic interactions with different resultant contributions to the volume of the mixtures. Weakening of interactions between molecules of cyclohexanone tends to increase in volume. Similarly, the disruption of alcohol multimers through breaking of hydrogen bonds makes a positive contribution to VE. The VE values of binary mixtures of cyclohexanone with alcohols follow the order, n-heptanol< n-octanol < iso-octanol. This may be due to the fact that the extent of hydrogen bonding and self association decreases with increasing chain length of alcohols. The weakening of interactions in iso-octanol when compared to n-octanol may be attributed to steric hindrance [22].
The positive values of VE over the entire range of mole fraction may be attributed to the dominance of molecular dissociation over association. Chowdhury et al [23] suggested for the mixtures of associated molecules that the main reason for a positive VE and a negative Δη may be the disruption of the associated molecules. The values of VE increase with increasing temperature of the mixture for all the three binary systems under study. The increase in VE is attributed to the breaking of donor-acceptor interactions between unlike molecules with a rise in temperature, leading to an expansion in volume that results in an increase in VE values [24].
The positive VE values for cyclohexanone + n-heptanol, cyclohexanone + n-octanol and cyclohexanone + iso octanol mixtures can be viewed in terms of fitting of smaller cyclohexanone (molar volume =1.039 x 10-5 m3mol-1) molecules into voids created by bigger n-heptanol (molar volume =1.425 x 10-5 m3mol-1), n-octanol (molar volume =1.592x10-5 m3mol-1) and iso-octanol (molar volume =1.579 x 10-5 m3mol-1) molecules, but this possibility seems to be dim. This may be due to the fact that alcohol molecules are highly self associated and well packed as indicated by high viscosity values (n-heptanol η= 5.0490cp, n-octanol η= 6.4020cp and iso octanol η= 6.9459cp) and very low values of free volume (n-heptanol Vf =1.8739 cm3mol-1, n-octanol Vf = 1.6073 cm3mol-1 and iso-octanol Vf =1.3640cm3mol-1) calculated by using the relation given by Suryanarayana, in the pure state, which might hinder the breaking of associated alcohol structures and do not allow the fitting of cyclohexanone molecules into the well packed structures of alcohol molecules, thereby, leading to positive VE values for all the mixtures.
The deviation in viscosity, Δη gives a quantitative estimation of intermolecular interactions. As suggested by Fort and Moore [25], the deviation in viscosity becomes positive as the strength of interaction increases. The Δη values may be generally explained by considering the following factors [26].
1. The differences in size and shape of the component molecules and the loss in dipolar interactions in pure components may contribute to a decrease in viscosity.
2. The specific interactions between unlike molecules such as hydrogen bond formation and charge transfer complexes may cause increase in viscosity in mixtures than in pure components.
The former effect produces negative deviation in viscosity while the later produces positive deviations. The net deviation in viscosity is generally considered as a result of the two major effects. The deviations in viscosity for the three systems at all the temperatures are negative indicating the dominance of nonspecific interactions between unlike molecules.
The experimental viscosities as a function of mole fraction of cyclohexanone for the three systems are shown in Figure 3(a - c). The values of Δη for all the binaries of cyclohexanone with n-heptanol, n-octanol and iso-octanol are negative over the entire composition range and a minimum around 0.5 mole fraction of cyclohexanone. This reveals that the strength of nonspecific interaction is not the only factor influencing the viscosity deviation of liquid mixtures. The molecular size and shape of the components also play an equally important role [27].
The negative Δη values for iso - octanol are larger than the other two systems to an appreciable amount. This seems to be due to the presence of methyl groups at different positions in aromatic solvent molecules offering resistance to flow process in binary mixtures making the flow process still more difficult causing an increase in Δη values for these solvents. The Δη values, thus have the following trend for binary mixtures with a common cyclohexanone:
n-heptanol < n-octanol < iso-octanol
This is also supported by the excess molar volume studies. As the temperature increases, the magnitude of the viscosity deviation sharply decreases due to the rapid breaking up of the molecular aggregates in the systems and ultimately tends to approach ideality.
From Figure 4 (a - c), ΔG*E is found to be negative in all the three systems. In the present investigation, it is found that the dipolar and dispersive forces are mainly operating in the behaviour of ΔG*E which supports Δη trends [28]. In order to elucidate the forces that are acting between unlike molecules, the help of excess Gibb's free energy of activation of viscous flow is quite essential. According to Reed and Taylor [29] and Meyer et al [30], positive Gibb's energy values indicate specific interactions while negative values indicate the dominance of dispersion forces.
Ultrasonic velocity, density and viscosity values were measured in binary liquid mixtures cyclohexanone with n-heptanol, n-octanol and iso-octanol at temperatures (303.15, 308.15, 313.15, 318.15) K for the entire mole fraction range. By using these experimental values, various parameters like adiabatic compressibility and molar volume were calculated and some exess parameters like excess adiabatic compressibility, βE, excess molar volume, VE, deviation in viscosity, Δη, and excess Gibb’s free energy of activation for viscous flow, ΔG*E are calculated. The positive values of βE, VE, and ΔG*E and negative values of Δη indicate that only dispersion and dipolar forces are operating with complete absence of specific interactions.
https://transplanthair.istanbul
https://hairclinicturkey.co
https://hairclinicistanbul.co
https://besthairtransplant.co
https://hairtransplantistanbul.co