ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
K.Padmaja1, Jyotsna Cherukuri2*, M.Anji Reddy3
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Water is the most ubiquitous liquid on our planet that is precious and vital to all life forms. The growth of industrialisation, urbanisation and a number of anthropogenic activities has made drinking water vulnerable and is posing a challenge to drinking water purification. In this paper a review of various purification methods are presented beginning with the conventional methods like activated carbon, activated alumina, silica, diatomaceous earth etc. to the latest techniques using nanomaterials, carbon nanotubes and nanocomposites. Break through techniques like thin films, quantum dots and aerogels in the purification of drinking water are cited. As each method has its own advantages and limitations in terms of removing contaminants, efficiency and cost effectiveness a blend of techniques is considered to be more beneficial than using a single technique.
Keywords |
| Adsorbents, activated carbon, diatoms, nano materials, carbon nanotubes, nano composites, thin films, quantum dots. |
INTRODUCTION |
| Water has always been an important and life-sustaining drink to humans and is essential for the survival of all known organisms. Though water is available in plenty, in the present day scenario availability of pure drinking water has become a rare commodity that is attributed to a number of reasons known. One of the most persistent problems affecting people throughout the world is insufficient access to clean water and sanitation. Each year, around 3.6 million people die because of issues related to contaminated water, poor hygiene, and unsanitary conditions. If those households, most at risk could gain access to safe drinking water, more than 2 million lives could be saved. Problems with water are expected to grow worse in the coming decades, due to the impact of growing industrialisation and urbanisation. This is leading to water scarcity globally, even in regions currently considered water-rich. To address these problems tremendous amount of research has to be conducted in identifying robust new methods of purifying water at lower cost and with less energy, while at the same time minimizing the use of chemicals and impact on the environment. Due to greater chances of water contamination in the supply systems, the US Environmental Protection Agency (EPA) is evaluating the use of a number of centralized water treatment concepts as „small system compliance technologyâ (USEPA, 1998). These include package treatment plants (i.e., factory assembled compact and ready to use water treatment systems), point-of-entry (POE) and point-of-use (POU) treatment units designed to process small amounts of water entering a given unit (e.g. building, office, household, etc.) or a specific tap/faucet within the unit [1]. Purification of water involves the removal of parasites, bacteria, algae, viruses, fungi, minerals (including toxic metals such as Lead, Copper, Arsenic etc.), and man-made chemical pollutants. Many contaminants can be dangerous, but depending on the quality standards, others are removed to improve the smell, taste, and appearance of water. In this paper a review of various technologies employed in improving the quality of drinking water are highlighted. |
I1 OVERVIEW OF METHODS OF PURIFICATION |
| Conventional water treatment may include chemical addition, coagulation, flocculation, sedimentation, filtration and disinfection, usually with chlorine [2].The drinking water treatment technologies used in the majority of systems include one or more of the processes using wide range of adsorbents, filtration techniques, electrical and disinfection methods, and latest technologies involving nanomaterials, carbon nanotubes, nano composites etc. as given in table-1. |
| Table-1. Various drinking water purification techniques: |
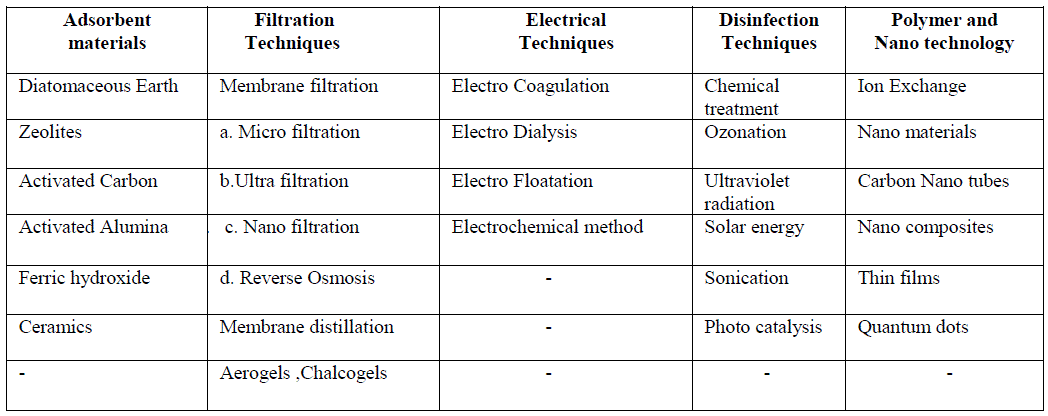 |
| All the above methods can be effectively used in the purification of drinking water. As each method can be used for the separation of one or few components, one particular method cannot be employed for the complete treatment of drinking water. Latest research includes usage of bio mass like banana, apple, tomato peels etc. in water purification. Various methods of purification of drinking water, their advantages, disadvantages and their use in latest technology are listed below. Adsorbents: Conventional drinking water purification methods involve a number of adsorbents of which most are natural materials. 1.1Diatomaceous earth or diatomite is a naturally occurring, soft, siliceous sedimentary rock formed by the deposition of hard-shelled unicellular algae called diatoms. The particle size ranges from 10 to 200 micrometers. The important use of diatomite is to filter water, particularly in the drinking water treatment process. It performs well on ground water with high concentration of iron and manganese and on surface waters with low influent turbidity, acceptable color and bacterial levels. Filtration through diatomaceous earth coated with cationic polyelectrolyte results in significant virus removal [3]. Zeta Plus filters composed of diatomaceous earth-cellulose-"charge-modified" resin mixtures and having a net positive charge efficiently adsorbed poliovirus from tap water at ambient pH levels 7.0 to 7.5[4].Though diatomite is a good adsorbent the only disadvantage of it is an increased risk of silicosis among workers in the crsistobalite DE industry who are exposed to high levels of airborne crystalline silica over decades. 1.2 Zeolites-Another natural material used in water purification is Zeolites which is micro porous, aluminosilicate minerals commonly known as “molecular sieves”. Their abundance and low cost is an added advantage. Various natural Zeolites around the world have shown varying ion-exchange capacity for cations such as ammonium and heavy metal ions and also anions and organics from aqueous solutions. Modification of natural zeolites can be done in several methods such as acid treatment, ion exchange, and surfactant functionalization, making them achieve higher adsorption capacity for organics and anion [5]. Synthetic zeolites are widely used in water purification. These zeolites are used as ion-exchange beds in domestic and commercial water purification, softening, and in other applications where molecules of certain sizes and shapes can pass through retaining the contaminants. |
| 1.3 Activated carbon is the most widely used adsorbent in water purification. The conventional centralized water treatment methods use activated carbon made from coal, wood, or coconut shell. Coconut shell is the most expensive and effective form. Carbon is “activated” by adding a positive charge, which enhances the adsorption and reduction of contaminants that have a negative charge. The three forms of activated carbon used in water filtration systems are granulated activated carbon (GAC), activated carbon block, and catalytic carbon. Activated carbon removes impurities, chemicals and contaminants from water through adsorption and a process called catalytic reduction. Contaminants removed include volatile organic compounds (VOCs), herbicides, pesticides, chlorine, radon, lead and most man-made chemicals. Activated carbon is not effective at removing heavy metals, nitrites, nitrates, dissolved inorganic contaminants or sediment. In water filters using carbon the characteristics of the carbon material (particle and pore size, surface area, surface chemistry, density, and hardness) influence the efficiency of adsorption [6]. This treatment can produce water of more desirable quality than that from some public or private supplies. The material in an activated carbon cartridge provides a growth surface for bacteria which may be a potential health hazard. The infusion of compounds such as silver can prevent bacterial growth on the carbon surface. Radon, which is a cause of lung cancer, is a radioactive decay product of natural uranium that moves through soil and rock into groundwater or enters a building through cracks or openings in the foundation. Radon is removed from water by granular activated carbon. The carbon, which is effective for many years, holds the radon until it decays into nonradioactive compound [7].Catalytic carbon, a new advanced activated carbon product, is designed to adsorb chloramines, an alternative to chlorine designed to inhibit the formation of carcinogenic trihalomethanes (THMs) caused by the interaction of chlorine with organic plant materials. Though activated carbon is a traditional water purification method but still it is most widely used and considered very effective method. 1.4 Activated alumina is a high surface-area, highly porous form of aluminum oxide that has a surface area significantly over 200 square meters/g. It is used as filter for fluoride, arsenic and selenium in drinking water. Excess of fluoride (>1.5 mg/l) in drinking water is harmful to the human health. Activated alumina filters can easily reduce fluoride levels from .5 ppm to less than .1 ppm [8]. The amount of fluoride leached from the water depends on the time of contact of the water and the alumina filter media. Basically, the more alumina in the filter, the less fluoride will be in the final, filtered water. Lower temperature water, and lower pH water (acidic water) are filtered more effectively too. Ideal pH for treatment is 5.5 which allows for up to a 95% removal rate. The ability of the alum-impregnated activated alumina (AIAA) for removal of fluoride from water through adsorption has been investigated by Tripathi, Jean and Gopal .Its efficiency to remove fluoride from water is found to be 99% at pH 6.5, contact time for 3 h, dose of 8 g/l, when 20 mg/l of fluoride is present in 50 ml of water. Energy-dispersive analysis of X-ray shows that the uptake of fluoride at the AIAA/water interface is due to only surface precipitation [9]. 1.5 Granulated ferric hydroxide (GFH) is an important method in the removal of arsenic [10]. Arsenic contamination of surface and subsurface waters is reported in many parts of the world and is considered a global issue. Arsenic enters into aquifers and wells through natural processes, and to the water cycle due to anthropogenic activities. Iron oxide adsorption is a water treatment process that is used to remove arsenic from drinking water which involves the arsenic species adsorption onto iron oxides. Ingestion of inorganic arsenic can result in both cancer (skin, lung and urinary bladder) and non-cancer effects. Granular ferric hydroxide is also a promising adsorbent for chromium removal, even in the presence of other interfering compounds, because granular ferric hydroxide treatment can easily be accomplished and removal of excess iron is a simple method for conventional water treatment plants. Thus, this method is regarded as a safe and convenient solution to the problem of chromium-polluted water resources as suggested by Asgari [11].Tap water seeded with different microorganisms or untreated waste water when passed through columns containing sand modified with a combination of ferric and aluminum hydroxide removes greater than 99% of Escherichia coli, Vibrio cholera, poliovirus 1 and coli phage MS-2 from dechlorinated tap water [12]. 1.6 Ceramics-Water filtration by using ceramics is an inexpensive and effective type of filtration method that relies on the small pore size of ceramic material to filter dirt, debris, and bacteria out of water. Typically bacteria, protozoa, and microbial cysts are removed but the filters are not effective against viruses since they are small enough to pass through to the other "clean" side of the filter. Recent studies show that metal oxide-enhanced ceramic surfaces can capture and inactivate virus indicators in a wide range of waters. Ceramic filtration does not remove chemical contaminants per se. However a high-performance activated carbon core inside the ceramic filter cartridge reduces organic & metallic contaminants. The active carbon absorbs chemicals like chlorine. Ceramic when combined with silver impregnated carbon is called sterasyl that is ideal for filtering microbiologically unsafe water; however sterasyl does not remove fluoride. The only disadvantage of ceramic materials is the brittle nature which may develop hairline cracks during handling. 2. Filtration techniques: Filtration is commonly the mechanical or physical operation which is used for the separation of solids from fluids (liquids or gases) by interposing a medium through which only the fluid can pass. A wide range of mediums are being employed of which membrane filtration is gaining importance in recent years. California in the US supplies drinking water by membrane filtration through public water supply systems. 2.1. Membrane filtration. Membrane filters are widely used for filtering both drinking water and sewage. For drinking water, membrane filters can remove virtually all particles larger than 0.2 um including Giardia and Cryptosporidium. However no filtration can remove substances that are actually dissolved in the water such as phosphorous, nitrates and heavy metal ions. Depending on the size of the particles removed, membrane filtration can be categorized into nano filtration, ultra filtration, microfiltration and reverse osmosis. As suggested by Bruggen & others in these pressure-driven membrane processes[13] a pressure exerted on the solution at one side of the membrane serves as a driving force to separate it into a permeate and a retentate. The permeate is usually pure water, whereas the retentate is a concentrated solution that must be disposed of or treated by other methods. Membranes used may be polymeric, organo-mineral, ceramic, or metallic, and filtration techniques differ in pore size, from dense (no pores) to porous membranes. Depending on the type of technique, salts, small organic molecules, macromolecules, or particles can be retained, and the applied pressure will differ.The removal of suspended solids and colloids can be achieved by using ultra filtration (UF). Nanofiltration (NF) allows separating the divalent cations (water softening), NOM (natural colour, trihalomethanes precursors), and results in the reduction of total dissolved solids (TDS) [14]. Reverse osmosis (RO) process demonstrates the best overall removal of TDS and organic compounds. High purity water can be successfully prepared by membrane distillation (MD). The important applications of MD process can be found in the technology of water treatment, seawater desalination and the concentration of a aqueous solutions [15]. Membranes can also be prepared from inorganic materials such as ceramics or metals. Ceramic membranes are micro porous, thermally stable, chemically resistant, and often used for microfiltration [16]. However, disadvantages such as high cost and mechanical fragility have hindered their wide-spread use. Metallic membranes are often made of stainless steel and can be very finely porous. Their main application is in gas separations, but they can also be used for water filtration at high temperatures or as a membrane support. MF/UF is seriously considered in most new WTP and expansion projects. However, major problems still needing attention are membrane fouling and membrane chemical stability. Being economical, environment friendly, versatile, and easy to use, membranes are a leading choice for water purification applications. a. Microfiltration: It is a membrane technical filtration process which removes contaminants from a fluid (liquid & gas) by passage through a micro porous membrane having micrometer sized filters. These filters are porous and allow water, monovalent species, dissolved organic matter, small colloids and viruses through but do not allow particles, sediment, algae or large bacteria through. A typical microfiltration membrane pore size range is 0.1 to 10 micrometers (μm). It is increasingly used in drinking water treatment, and effectively removes major pathogens and contaminants such as Giardialamblia cysts, cryptosporidium ocysts, and large bacteria. For this application the filter has to be rated for 0.2 μm or less. Microfiltration membranes were first introduced to the municipal water treatment market in 1987 and applied primarily to waters that were relatively easy to treat. These systems are designed to remove suspended solids down to 0.1 micrometers in size, in a feed solution with up to 2-3% in concentration. Microfiltration is fundamentally different from reverse osmosis and nanofiltration because these systems use pressure as a means of forcing water to go from low pressure to high pressure. Microfiltration can use a pressurized system but it does not need to include pressure [17]. b.Ultrafiltration-With its unparalleled reliability and ability to remove particles in the nano-range, ultrafiltration (UF) has become the treatment technology, for drinking water treatment. Most common UF membranes used for water filtration are manufactured as hollow fibres, with an inner diameter of typically 0.7 to 0.9 mm. Ultrafiltration membranes use polymer membranes with chemically formed microscopic pores that can be used to filter out dissolved substances avoiding the use of coagulants. Unlike reverse osmosis (RO), UF does not change the mineral content of the water significantly and it can operate at pressures as low as 4 to 14 psi. Classical membrane processes like RO start to work efficiently only at around 100 psi. The Greatest advantage of Ultrafiltration is an absolute barrier for bacteria, virus and parasites. Combined with activated carbon prefiltration, it removes taste, odour, pesticides and residuals of antibiotics. It requires low filtration pressure and low energy consumption and is a complete green technology. UF can also be directly operated on solar power as suggested by Michael and Dan [18]. C.Nanofiltration is a very inexpensive method compared to conventional treatment systems. It is a relatively recent membrane filtration process used most often with low total dissolved solids water such as surface water and fresh groundwater, with the purpose of softening (polyvalent cation removal) and removal of disinfection by-product precursors such as natural organic matter and synthetic organic matter [19][20]. Nano porous membranes are suitable for a mechanical filtration with extremely small pores smaller than 10 nm. On a larger scale, the membrane filtration technique is named ultrafiltration, which works down to between 10 and 100 nm. They exhibit performance between that of RO and UF membranes. Dendritic polymers are soft nanoparticles, with sizes in the range of 1–20 nm, exhibit many features that make them particularly attractive as functional materials for water purification [21]. D.Reverse osmosis- Reverse osmosis is one of the most common and effective water treatment systems. This water treatment process is also pressure driven membrane process. RO filters, removes ionized salts, colloids, and organic molecules down to a molecular weight of 100 .RO membranes are made of a thick polyamide film that contains tiny pores through which water can flow. The pore sizes can vary from 0.1 to 5,000 nanometers (nm) depending on application. These pores are small enough to restrict organic compounds such as minerals and salt, but allow water molecules to pass through. These are restrictive enough to filter out disease causing pathogens and bacteria from water. Reverse osmosis is highly effective in removing several impurities from water: total dissolved solids (TDS), turbidity, asbestos, lead and other heavy metals, radium, and many dissolved organics. About 31 out of 35 American cities are found to have chromium 6 which is carcinogenic in their drinking water. Removal of Chromium-6 from water is achieved with a reverse osmosis system [22]. In addition to that it will also remove arsenic, barium, copper, lead and fluoride. The main disadvantage of this system is the wastage of large amount of water, about 3 to 9 gallons of water per gallon of purified water produced. Secondly, reverse osmosis treats water slowly, it takes about 3 to 4 hours for a residential RO unit to produce one gallon of purified water. Though reverse osmosis membranes remove about 99% of the solutes, but the concentrations of essential nutrients, such as calcium and magnesium ions, are reduced to levels that are below the specifications of the World Health Organization standard for drinking water. |
2.2. Membrane distillation |
| Another promising and efficient water purification method is membrane distillation (MD) which is a thermal, vapordriven transportation process through micro porous and hydrophobic membranes. MD is applied as a non iso thermal membrane process in which the driving force is the partial pressure gradient across a membrane that is porous, not wetted by the process liquid. The passing vapor is then condensed on a cooler surface to produce freshwater. The commercially available membranes are made mainly of polypropylene (PP), polytetrafluoroethylene (PTFE), polyvinylidene fluoride (PVDF), and polyethylene (PE). The potential applications of MD are production of high purity of water, concentration of ionic, colloid or other nonvolatile aqueous solutions, and removal of trace volatile organic compounds from wastewater [23]. The waste heat produced can be reused. Hence, MD is a promising, yet still emerging technology for water treatment. The various properties of different filtration methods are listed below in table 2 [24]. |
| Table 2- Properties of membrane filtration methods: |
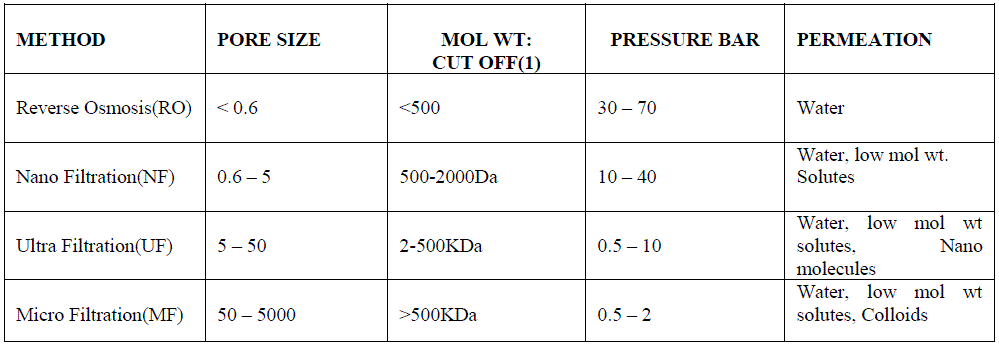 |
| (1) Molecular Weight (Dalton) cut off of the membrane where solutes of this weight are rejected by 90% |
| Other promising techniques in water purification are Aerogels. These are synthetic, porous ultra-light material derived from a gel, in which the liquid component of the gel has been replaced with a gas. A chalcogel is an aerogel made from chalcogens which preferentially absorb heavy metals, showing promise in absorbing pollutants mercury, lead, and cadmium from water [25].Recent studies revealed that Carbon nanotube–graphene hybrid aerogels show very promising performance in water purification including capacitive deionization of light metal salts, removal of organic dyes and enrichment of heavy metal ions [26].The carbon aerogels are called super sponges as they can absorb organic solvents, oils etc. 900 times their weight. 3. Electro Chemical techniques A number of Electro Chemical techniques are employed in drinking water purification. Some of the important ones are Electro coagulation, Electro dialysis, and Electro Floatation. 3.1.Electrocoagulation present a robust, novel and innovative method in which a sacrificial metal anode corrodes, due to an applied electric potential, with the simultaneous evolution of hydrogen at the cathode which is removed by flotation. This has the major advantage of providing active cations required for coagulation, without increasing the salinity of the water [27].This process is mostly used in waste water treatment in the removal of heavy metals [28] fluoride ions etc. but increasingly is applied to drinking water treatment due to its high efficiency, cost effectiveness and ecofriendly nature. Electro coagulation coupled with Microfiltration (EC-MF) process is a highly effective water treatment technique. The factors such as current density, electrolytic time and pH value influences the removal efficiencies of TOC, NH3-N and oil [29]. These pollutants decreased with increasing current density. Arsenic can be removed from drinking water using electro coagulation (EC) with Iron electrodes followed by filtration. A sand filter is used to remove flocs generated in the EC process. The EC process decreases the residual arsenic concentration to less than 10 μg L−1 [30]. 3.2. Electro Dialysis (ED) is an electrically driven membrane separation process that is capable of transporting ions through semi permeable membrane. The membranes are cation or anion selective hence charged particles only can be removed. It is an efficient process for the treatment of drinking water with high nitrate concentration. However it achieves only a transfer of pollution by producing concentrated brines. A study by Allain, Christelle and others demonstrated the feasibility of ED brine denitrification in a membrane bioreactor (MBR)[31]. 3.3. Electro Floatation (EF) technology is effective in removing colloidal particles, oil & grease, as well as organic pollutants from water. It is proven to perform better than either dissolved air flotation, sedimentation, or impeller flotation (IF). The separation of the flocculated sludge from the treated water can be accomplished by using EF. 4. Disinfection methods Disinfection is an important step in ensuring that water is safe to drink. Water systems add disinfectants to destroy microorganisms that can cause disease in humans. Primary methods of disinfection are chlorination, chloramines, ozone, and ultraviolet light. Other disinfection methods include chlorine dioxide, potassium permanganate, nanofiltration, solar radiation and sonication.. Conventional disinfection methods leads to the formation of disinfection by products and hence paved way to consider innovative approaches that enhance the reliability and robustness of disinfection while avoiding DBP formation. 4.1. Chemicals like Chlorine gas are very effective for removing almost all microbial pathogens and is appropriate as both a primary and secondary disinfectant. It is released from a liquid chlorine cylinder and is lethal at concentrations as low as 0.1 percent air by volume. Other chlorination methods involve the addition of sodium and calcium hypo chlorites but these are corrosive in nature. Chloramine is an effective bactericide that produces fewer disinfection byproducts but is a weak disinfectant. The main disadvantage of chemical disinfection is the formation of halogenated byproducts which are carcinogenic. To control their formation EPA has identified three strategies involving the removal of the by-products after they are formed, using alternate disinfectants and lastly reducing the concentration of organics in the water before oxidation or chlorination[32]. 4.2. Ozonation: Ozonation is a good disinfection method to convert impure water into to pure drinking water. It inactivates and destroys all bacteria, virus and protozoa. Ozone oxidizes both organic and inorganic substances, remove unwanted taste, odour and color, and provide effective disinfection. Ozone acts over 3000 times faster than chlorine, requiring shorter contact time and dosage than chlorine with the ability to kill 99% of all waterborne bacteria, germs, viruses and most pesticides by rupturing the cells of micro-organisms, or destroying odors and chemicals by oxidation. |
| Ozone is an effective air and water disinfectant, but high concentrations are harmful to living tissues. While the use of ozone for most drinking water systems is unnecessary and impractical due to municipal chlorination, it is highly recommended method of sterilization for water that is microbiologically unsafe to drink, as an alternative to UV-C sterilization. 4.3. Ultraviolet radiation: Investigations by various laboratories in the recent years proved that biological resistant pollutants in water can be decomposed by UV light, solar energy in the presence of special catalysts like Tio2 and ionizing radiation like high energy electrons or Y-rays [33].UV disinfection is increasingly used for drinking water treatment due to its effectiveness against cyst forming protozoa such as Giardia and Cryptosporidium. But some pathogenic viruses such as adenoviruses are highly resistant to UV disinfection, requiring very high dosages [34].A combination of UV with photo catalytic nanomaterial like TiO2 and Fullerol provides additional inactivation.UV reactors internally coated with TiO2 can enhance the disinfection rate [35]. UV radiation effectively destroys bacteria and viruses but is unsuitable for water with high levels of suspended solids, turbidity, colour, or soluble organic matter. 4.4. Solar water disinfection is a water purification that uses solar energy, in one or more ways, to make contaminated water safe to drink by ridding it of infectious disease-causing biological agents such as bacteria, viruses, protozoa and worms. However, disinfection may not make all kinds of water safe to drink due to non-biological agents such as toxic chemicals or heavy metals. Consequently, additional steps beyond disinfection may be necessary to make water clean to drink. There are three primary subsets of solar water disinfection- using electricity, heat and ultraviolet radiation. Solar thermal water disinfection uses heat from the sun to heat water to 700C-1000C for a short period of time. Solar ultraviolet water disinfection, also known as SODIS, is a method of disinfecting water using only sunlight and plastic PET bottles. SODIS is a free, simple, environmentally sustainable, low-cost solution for drinking water treatment at household level for people consuming microbiologically contaminated raw water and effective method for decentralized water treatment, and is recommended by the World Health Organization as a viable method for household water treatment and safe storage [36]. Plastic bottles are made of either PET (Poly Ethylene Terephtalate) or PVC(PolyVinylChloride) which contain additives like UV-stabiliser to increase their stability or to protect them and their content from oxidation and UV radiation [37].The limitation of SODIS is that it cannot be used for turbid water, additional filtering is then necessary. 4.5. Sonication- is a process of disinfection using sound waves. Ultrasound (sound waves of a frequency higher than 20 kHz) can lead to structural and functional disruption of cyanobacterial cells [38] and its use as a treatment option to control cyanobacterial blooms has been under consideration in recent decades. Low frequencies are desirable due to relatively lower power consumption however, previous studies show a trend of higher frequencies being more effective for cyanobacterial inhibition than lower frequencies [38][39].The development of bacterial blooms in water bodies imparts undesirable characteristics to the water such as odors, tastes and the potential presence of toxins. Several chemical and physical methods have been used to control the blooms, but have limitations in terms of pollution and application on a large scale. A more recent approach has been the use of sonication in the control of cyanobacteria (also referred to as blue green algae) [40]. Other indicators of water quality such as suspended solids (SS), chemical oxygen demand (COD), transparency and total phosphate concentration were found to be significantly lower on sonication. The major advantage of sonication is that it is environmental friendly compared to treatment strategies such as the use of algaecides. |
5. Polymer and Nano technology |
| 5.1. Ion-exchange process- is an exchange of ions between two electrolytes or solutions generally used in water purification .Polymers are being extensively used in water purification either as membranes or as resins. Certain ions like nitrates present in water increases aquatic plant growth and bring changes in the types of plants and animals that live in the stream. This, in turn, affects dissolved oxygen, temperature, and other indicators. Ion-exchange method can be employed to remove nitrate ions successfully. Nitrate is removed by chloride ion-exchange, and the strong-base anion resin is completely regenerated at mild reaction conditions (i.e. ambient temperature, atmospheric pressure) in a closed circuit containing a single-flow fixed-bed reactor packed with a Pd–Cu/γ–Al2O3 catalyst [41].Ion exchange process with organic and inorganic ion exchangers efficiently removes harmful transition metals like As, Mn, Cr etc. from water. Cations like calcium, magnesium and ammonium and anions like nitrates, bromides, sulphates can be easily removed .This process coupled with adsorbents like activated carbon increases the efficiency. |
| 5.2. Nanotechnology has spurred significant interest in the environmental applications of nanomaterial. Nanomaterials are excellent adsorbents, catalysts, and sensors due to their large specific surface area and high reactivity. More recently, several natural and engineered nanomaterials have also been shown to have strong antimicrobial properties that include silver nanoparticles, photo catalytic TiO2, fullerol and carbon nanotubes. a) Nanoparticles can be used to convert the contaminating chemical in water through a chemical reaction to make it harmless. Studies have shown that this method can be used successfully to reach contaminates dispersed in underground ponds and at much lower cost than methods which require pumping the water out of the ground for treatment .Another challenge is the removal of salt or metals from water. A deionization method using electrodes composed of nano-sized fibres shows promise for reducing the cost and energy requirements of turning salt water into drinking water. Standard filters do not work on virus cells. Therefore a filter only a few nanometres in diameter is currently being developed that should be capable of removing virus cells from water. Nanotechnology provides a number of applications in water purification and a few of them are: nanoscavengers like silver nanoparticles, destroy the bacteria in water and when a magnetic field is applied the nanoscavengers are removed from the water. Pellets containing nanostructured palladium and gold as catalysts are used to breakdown chlorinated compounds contaminating ground water. These pellets allow almost every atom to react with the chlorinated compounds, reducing the cost of the treatment. A strip of glass covered in hairy nanoparticles (nano-hair) can cheaply and conveniently measure mercury pollution in water, which attacks the nervous system, and other toxic metals in fluids [42]. b) Nanocomposites- Researcher Thalappil Pradeep, a materials scientist at the Indian Institute of Technology Madras, and his team created a unique family of nanocrystalline metal oxyhydroxide-chitosan granular composite materials. This material, which forms a cage-like matrix, strongly bonds to embedded nanoparticles. The nanoparticles allow only ions to escape at a controlled rate. These ions then kill microbes found in the water, without releasing nanoparticles. “For a diverse array of contaminants, you can use a variety of nanostructured materials to finally get water that is purified”. The purifiers would cost small families about US$2.50 per year [43]. c) Carbon nanotubes- In recent years Carbon nanotubes (CNT) have received special attention for its exceptional water treatment capabilities and proved to work effective against both, chemical and biological contaminants. CNTs as an adsorbent media, able to remove wide range of contaminant heavy metals such as Cr3+, Pb2+,and Zn2+ metalloids such as arsenic compounds ,organics such as polycyclic aromatic organic compounds (PAH) ,atrazine, and a range of biological contaminants including bacteria ,viruses and cyanobacterial toxins. The success of CNTs as an adsorbent media in the removal of biological contaminants, especially pathogens is mainly attributed to its unique physical, cytotoxic and surface functionalizing properties [44].Carbon nanotube (CNT) adsorption technology has the potential to support point of use (POU) based treatment approach for removal of bacterial pathogens, natural organic matter (NOM), and cyanobacterial toxins from water systems. Unlike many micro porous adsorbents, CNTs possess fibrous shape with high aspect ratio, large accessible external surface area, and well developed mesopores; all these contribute to their multifold efficiency. 5.3 Thin Films Gurpreet Singh, a Ph.D. candidate in the University of Akron College of Polymer Science and Polymer Engineering, led a team of researchers to devise a method that enables the films to assemble themselves and allow them to serve as templates or directly as end products. The films can be embedded with nanoparticles that enable everything from data storage to water purification. Thin films serve as another potential water treatment technology. TiO2 thin Film photo catalysts show bactericidal and detoxification effects in E.Coli present in water [45]. Thin film Nano composites are used as membranes in drinking water purification which exhibits greater stability and foul resistance. 5.4 Quantum dots Alexey I. Ekimov and Louis E. Brus discovered Quantum dots which are few nanometers in size. These are more closely related to individual atoms and are called artificial atoms. Recent studies have shown that quantum dots can be used in water treatment as sensors and photocatalysts.Semiconductor quantum dot-conjugated antibodies were successfully developed to label Cryptosporidium parvum and Giardia lamblia. This novel fluorescence system exhibited superior photo stability, gave 1.5- to 9-fold-higher signal-to-noise ratios than traditional organic dyes in detecting C. parvum, and allowed dual-color detection for C.parvum and G.lamblia [46].Highly luminescent semiconductor quantum dots of Cd-Se,ZnS, can be used as fluorescent sensors or probes for the detection of various contaminants in drinking water. CdTe quantum dots are used in the detection of harmful metals like As, Cr, Pb etc.[47]. Various purification methods and the contaminants removed along with their efficiency and cost effectiveness are summarized in table-3. |
| Table. 3.Purification methods and the contaminants removed. |
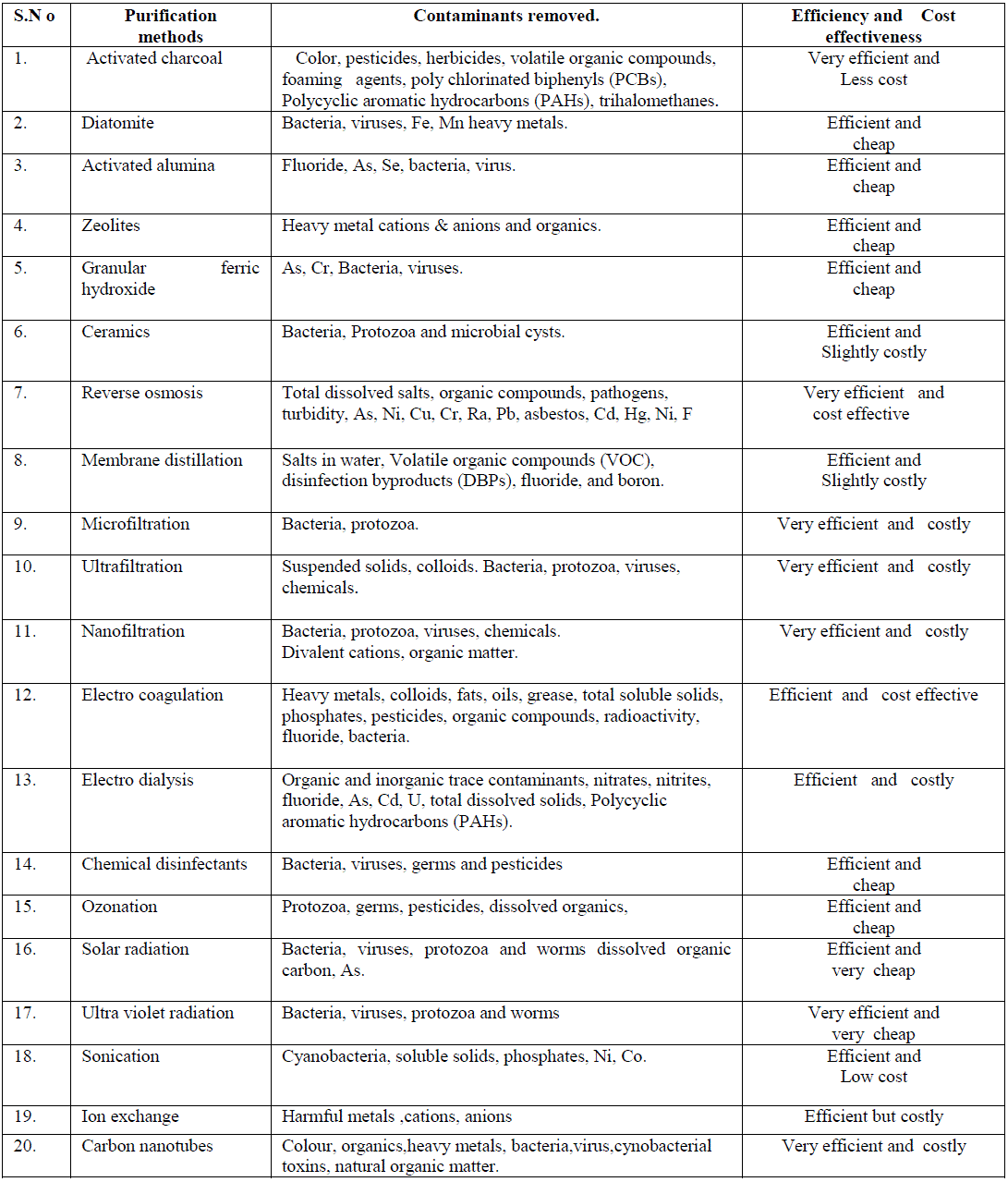 |
III. CONCLUSION AND FUTURE PERSPECTIVES |
| The impact of industrialization and urbanization on the quality of drinking water has been rising alarmingly in the recent years. Availability of pure and contaminant free water has become a rare phenomenon. All treatment methods have limitations and often a combination of techniques is required to effectively treat water. With the advent of technology a number of novel, efficient and cost effective methods for the purification of drinking water have come up. Purification at the point of entry and point of use is considered to be more advantageous than centralized system to ensure greater safety of drinking water. |
ACKNOWLEDGEMENTS |
| The author is grateful to her institution Vignan Jyothi Institute of Engineering and Technology for creating a scientific environment congenial for pursuing research. The author profoundly thanks her guide and mentor Jyotsna Cherukuri and Prof.M.Anji Reddy for providing the required guidance and motivation in writing the paper. |
References |
|