ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Asama. N. Naje1 , Azhar S.Norry2, Abdulla. M. Suhail3
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Tin Oxide (SnO2) nanoparticles powder have been synthesized by chemical precipitation method. The samples were characterized by X-ray diffraction, UV-Visible absorption and scanning probe Microscope SPM. The Xray analysis shows that the obtained powder is SnO2 with tetragonal rutile crystalline structure and the crystalline size in the range of 8-10nm. The SPM investigation reveals that the average particles size is 73nm. The optical band gap values of SnO2 nanoparticles were calculated to be about 4.3eV in the temperature 550 o C, comparing with that of the bulk SnO2 3.78eV, by optical absorption measurement
Keywords |
| SnO2 nanoparticles, X-ray diffraction ,Morphology, Optical Properties. |
I. INTRODUCTION |
| Nanometer-sized materials have recently attracted a considerable amount of attention due to their unique electrical, physical, chemical, and magnetic properties, these materials behave differently from bulk semiconductors. With decreasing particle size the band structure of the semiconductor changes; the band gap increases and the edges of the bands splits into discrete energy levels. These so-called quantum size effects occur [1-5]. These quantum size effects have stimulated great interest in both basic and applied research. |
| Tin oxide (SnO2) is one of the most intriguing materials to be investigated today, This is because tin dioxide is a well-known n-type semiconductor with a wide band gap of 3.6-3.8 eV [ 6-8], and for its potential application in transparent conductive electrode for solar cells a gas sensing material for gas sensors devices, transparent conducting electrodes, photochemical and photoconductive devices in liquid crystal display , gas discharge display, lithium-ion batteries, etc[9- 14 ]. |
| Many processes have been developed to the synthesis of SnO2 nanostructures, e.g., spray pyrolysis, hydrothermal methods, chemical vapor deposition, thermal evaporation of oxide powders and sol–gel method [15-20 ] |
| In the present work the fabrication and characterization of crystalline SnO2 nanoparticles powder by chemical precipitation method was studied |
II. EXPERIMENTAL WORK |
| SnO2 nanopowders were prepared by means of dissolving of 2 g (0.1 M) stannous chloride dehydrate (SnCl2.2H2O) in 100 ml distilled water. After complete dissolution, ammonia solution was added to the above solution by drop wise under stirring. The resulting gels were filtered and dried at 80ºC for 24 hours in order to remove water molecules. Finally, tin oxide nanopowders were formed at 550ºC for 2h. |
| The obtained samples were characterized by X-ray powder diffraction (XRD) using (XRD -6000), supplied by SHIMADZU. The surface morphology of the samples was observed by Scanning probe Microscope (SPM) by using CSPM AA3000, supply by Angstrom Company. Optical absorption spectra of the samples were taken with OPTIMA SP-3000 UV-VIS Spectrometer,. The room temperature photoluminescence (PL) spectra of SnO2 were recorded with SL 174 SPECTRFLUORMETER. |
III. RESULTS AND DISCUSSION |
| A. Characterization of the SnO2 powder |
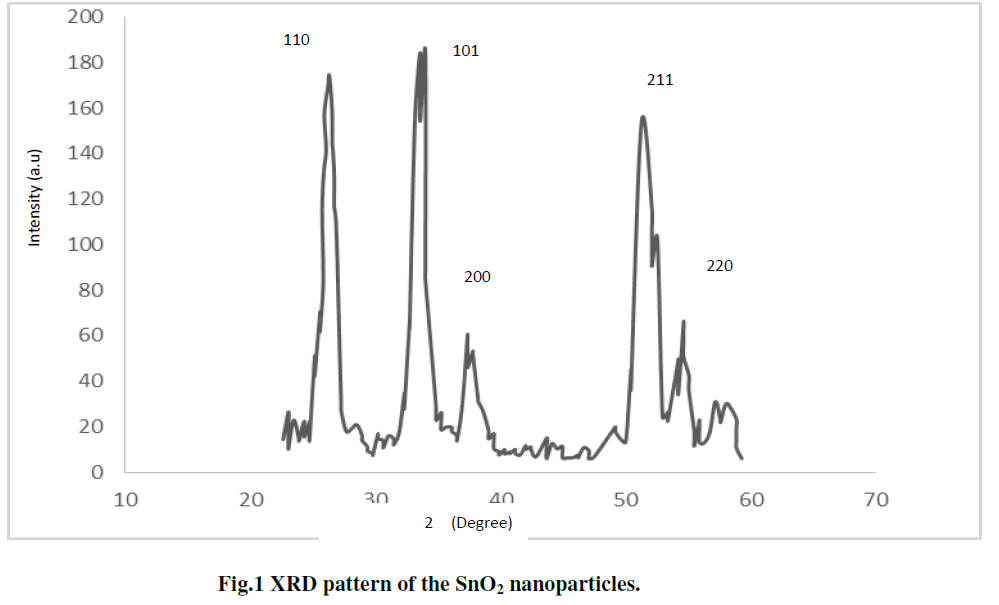
| The x-ray diffraction (XRD) pattern of SnO2 nanoparticles powder is shown in Fig. 1 The peaks at 2θ values of 26.6°, 33.8°, 37.9°, 51.8°, and 54.7° can be associated with (110), (101), (200), (211) and (220) respectively. The SnO2 product shows tetragonal structure, which are in good agreement with other literatures [21-22]. The average particle size (D) was determined using the Scherer,s eq. 1 [ 23]: |
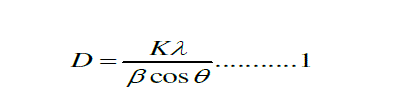 |
| Where D is the crystallite size, K is the shape factor, being equal to 0.9 , λ is the X-ray wavelength, β is the full width at half maximum of the diffraction peak, and ÃÂ is the Bragg diffraction angle in degree. The average particles size was found to be in the range of 8-10nm [7 ]. |
| B. Morphology of SnO2 Nanoparticles |
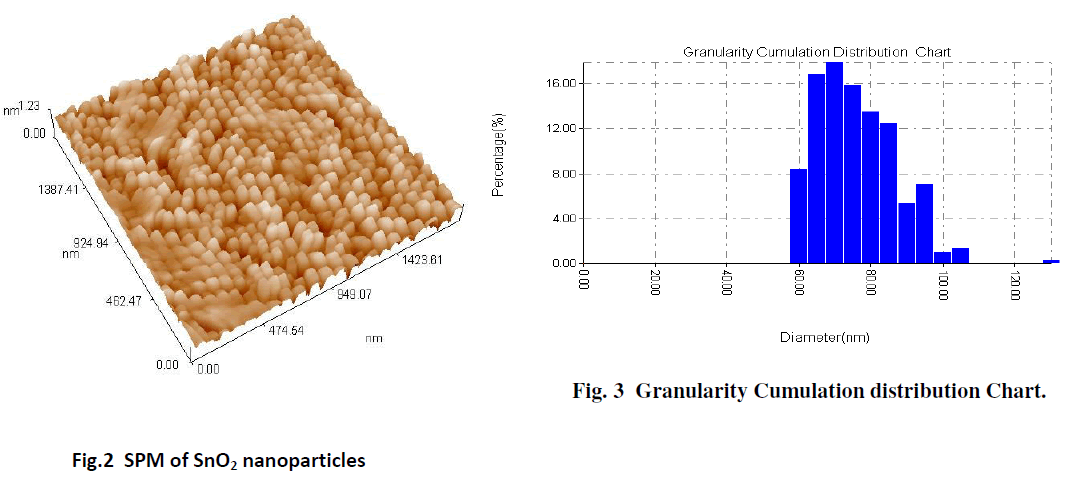
| The SPM image of the surface morphology of the SnO2 film gives a good indicator for formation of the SnO2 nanoparticles. The average particle size determined from SPM, is about 73.65 nm, as shown in Fig. 2 The results are obtained from the SPM of the SnO2 nanoparticles show that the histogram of the percentage of SnO2 as a function of the grain size as in the Fig. 3. |
 |
 |
| C. Optical properties |
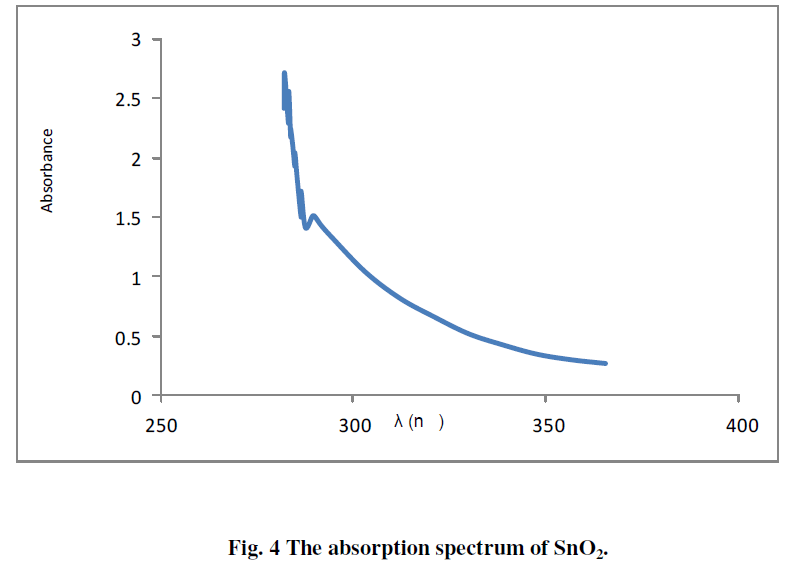
| The absorption spectrum of SnO2 deposited on glass substrate is shown in Fig. 4. The figure shows high absorption coefficient in the UV region, whereas it's transparent in the visible region. |
 |
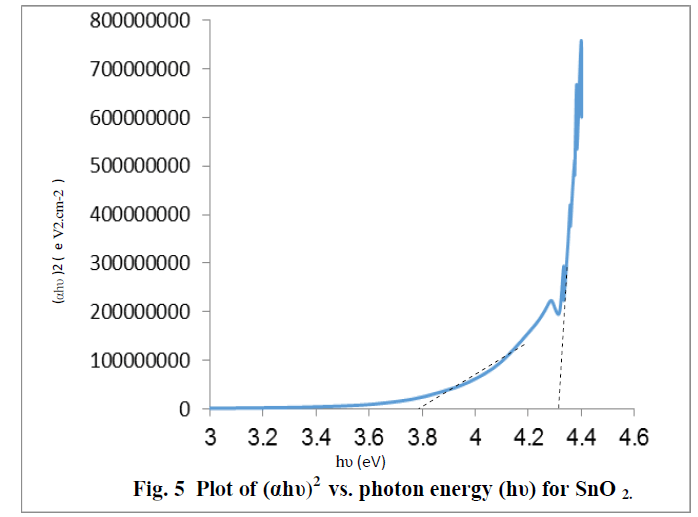 |
| The optical band gap energy (Eg) of the semiconductor is calculated from Tauc relation [ 24]. A plot of (ï¡hï®)2 versus hυ shows intermediate linear region, the extrapolation of the linear part can be used to calculate the Eg from intersect with hï® axis as shown in Fig. 5 .The resultant values of Eg for SnO2 is found to be about 3.78eV and 4.3 eV[25] , The above two values may be related to the formation of nanostructures of SnO2 and the bulk SnO2, these values show a good agreement with the values published by other workers. [8,18] |
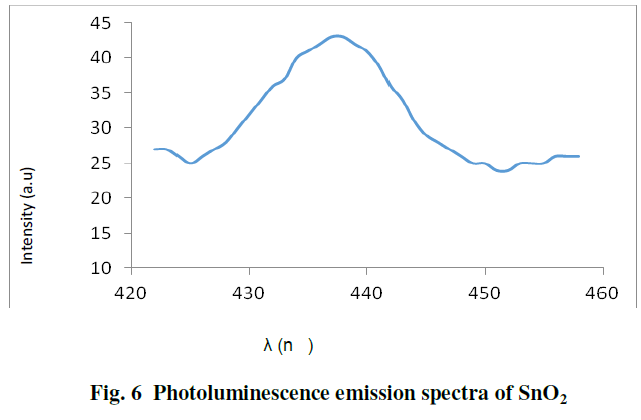
| Fig. 6 shows the photoluminescence emission spectra of SnO2 nanoparticles at 280nm excitation, SnO2 nanoparticles exhibit emission at 437nm. The emission maximum of 437 nm is lower than the band gap of the SnO2 bulk, this peak can be attributed to the contribution of oxygen vacancies and defect in the SnO2 nanoparticles [ 4,8 ]. |
 |
IV.CONCLUSIONS |
| By chemical precipitation method SnO2 nanoparticles powder were synthesized at 550 oC . The structural, morphological and optical properties of a SnO2 sample were investigated The XRD pattern of the prepared sample is indexed to the tetragonal structure of SnO2, and the calculated particle size in the range 8-10nm. The average particle size (73nm) of the SnO2 nanoparticles, estimated by SPM. The optical bandgap of the SnO2 were found to be 3.78 eV and 4.3eV. The photoluminescence emission spectra of SnO2 nanoparticles at 280nm excitation, exhibit emission at 437nm. |
References |
|