e-ISSN: 2322-0139 p-ISSN: 2322-0120
e-ISSN: 2322-0139 p-ISSN: 2322-0120
Department of Pharmacology, Karnataka college of Pharmacy, Bangalore-560064, Karnataka, India
Received date: 17 May 2013 Accepted date: 07 June 2013
Visit for more related articles at Research & Reviews: Journal of Pharmacology and Toxicological Studies
To study the antitumor effect of Kigelia africana. Antitumor activity of methanolic extracts of 100, 200 mg/kg of Kigelia Africana leaves was evaluated against Ehrlich ascites carcinoma (EAC) tumor in mice. Acute and short-term toxicity studies were performed initially in order to ascertain the safety of methanolic extracts of Kigelia Africana. After 12 days of tumor inoculation, the extract was administered daily for 30 days. The effect of methanolic extracts of Kigelia Africana on the growth of tumor, life span of EAC bearing hosts and simultaneous alterations in the haematological profile and histopathological profile were estimated. The methanolic extracts of Kigelia africana showed decrease in tumor size, average body weight, mean survival time thereby increasing life span of EAC tumor bearing mice. Haematological profile reverted to more or less normal levels in extracts treated mice. Histopathology has minimal effects when compared but a significant variation is seen.
Kigelia Africana, Ehrlich’s ascites carcinoma, 5 FluoroUracil
Cancer is a cellular tumour that, unlike benign tumour cells, can metastasize and invade the surrounding and distant tissues [1]. Cancer is the abnormal growth of cells in our bodies that can lead to death. These cells are born due to imbalance in the body and by correcting this imbalance the cancer may be treated. The major causes of cancer are smoking, dietary imbalances, hormones and chronic infections leading to chronic inflammation. The important preventive methods for most of the cancers include dietary changes, stopping the use of tobacco products, treating inflammatory diseases effectively, and taking nutritional supplements that aid immune functions [2]. The eluted flavanoid has broad antibiotic properties – including nematocidal, antimicrobial, antiprotozoal and insecticidal activities. A thorough and complete literature search on Kigelia Africana Linn.was performed from the chemical abstracts, national and international journals, E-library, internet and other research materials. Kigelia Africana is a medicinal plant of the Bignoniaceae family. It is widely distributed in Eastern Ghats and Deccan plateau in India. The leaves of Kigelia Africana are used in traditional medicine for relieving headache, anti-inflammatory activity, preservative, anti-bacterial activity. General pharmacological studies revealed analgesic, inflammatory, anti- microbial activity, anti-malarial activity, anti-fungal activity, anti-ulcer activity of aqueous and methanolic extracts of the leaves Kigelia Africana. The anti-inflammatory [11] and wound healing [12] properties of the crude alcoholic extract of the leaves of Kigelia Africana in acute inflammation model has been reported. The phytoconstituents present in this plant are kigelinole, isokigelinole, pinnatal, isopinnatal, B-Sitosterol, ferulic acid and lapachol [3]. Based on this information the present study was carried out to evaluate the antitumor activity, status of methanolic extracts of Kigelia Africana against Ehrlich’s ascites carcinoma (EAC) in Swiss albino mice.
Plant Material and Extraction
Leaves of Kigelia Africana were collected in the month of June, 2012 from Tirupathi forest and it was identified and authenticated by K.Madhavachetty, Professor Department of Botany, Padmavathi College, Tirupathi and the voucher specimen was deposited in the Department of Pharmacology, Karnataka College of Pharmacy. The shed dried healthy leaves were powdered separately using mechanical grinder and then were passed through sieve in order to maintain uniform size.About 250 g of dry leaf powder was extracted using methanol solution in Soxhlet apparatus. After about 2hrs, the materials were concentrated by evaporation. The concentrated extracts were shade dried to remove the solvent for further use. The yield of extract was calculated and stored for further use. The extract was then subjected to column chromatography, from which a flavonoid was isolated and it was confirmed through spectroscopy and structural elucidation [4].
Cell Lines Used
Ehrlich Ascites cell lines were obtained from Amala cancer research institute, Thrissur and are used for the Invitro assay.
Experimental Animals
Female Swiss albino mice weighing between 18-25 gm were used for present study. They were maintained under standard environmental conditions and were fed with standard pellet diet of water. The mice were acclimatized and laboratory condition for 10 days before commencement of experiment.
Anti-Tumour Activity
Experimental Design
Female Swiss Albino mice divided into 4 groups (n=6). All the groups were injected with EAC cells (0.2 ml of 2 ×106 cells/mouse) sub-cutaneously at the abdomen region to cause mammary tumor. This was taken as day zero and the treatment starts on the twelfth day after induction of tumor cells. The parameters selected are checked accordingly for every five days. The extract is then administered to the mice except the control group. Standard group receives 5 Fluoro Uracil as standard drug. One group receives 100mg and the other receives 200mg of the extract. The parameters include the Mean survival time (MST), % increase in life span(%ILS), Body weight,tumor size, Haematological parameter, RBC, WBC, Haemoglobin, Histopathology of liver and kidney.
Mean Survival Time
Animals will be inoculated with EAC cells (1 X 106cells/mouse) on day ‘0’ and the median survival time (MST) of each group, consisting of 6 mice will be noted.
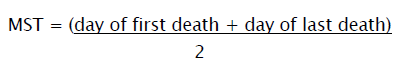
Percentage Increase Life Span (% ILS)
The effect of the drugs on tumor growth was monitored by recording the mortality daily for a period of 6 weeks and percentage increase in life span (%ILS) was calculated.
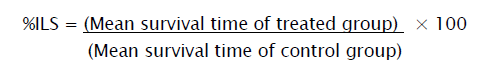
Body Weight
Body weights of the experimental mice were recorded both in the treated and control group at the beginning of the experiment (day 0) and sequentially on every 5th day during the treatment period.
Tumor Size
Tumour mass was measured from the 11th day of tumour induction. The measurement was carried out every 5th day for a period of 30 days. The volume of tumour mass was calculated using the formula:
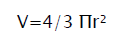
Where ‘r’ is the mean of ‘r¹’ and ‘r²’ which are the two independent radii of the tumour mass [7].
Effect on Haematological Parameters
At the end of the experimental period, 6 mice of each group were sacrificed the next day after an overnight fast by cervical dislocation.
Blood was collected by Retro-orbital route and used for the estimation Hemoglobin (Hb%) content, red blood cell count (RBC)8 and white blood cell count (WBC)9.
Histopathological Studies
A portion of liver and kidney of animals in all groups were stored in container for 12 hours in 10% formalin (10 ml of formaldehyde in 90 ml of normal saline) solution and subjected to histopathological studies.
Statistical Analysis
Values were represented as mean ± SEM. Data were analysed by one-way analysis of variance (ANOVA) followed by Dunnett’s test using statistical package for social sciences (SPSS) version 10.0. P<0.05 was considered significant. The toxic control group was compared with the normal control group and all other treatment groups were compared with the toxic control group.
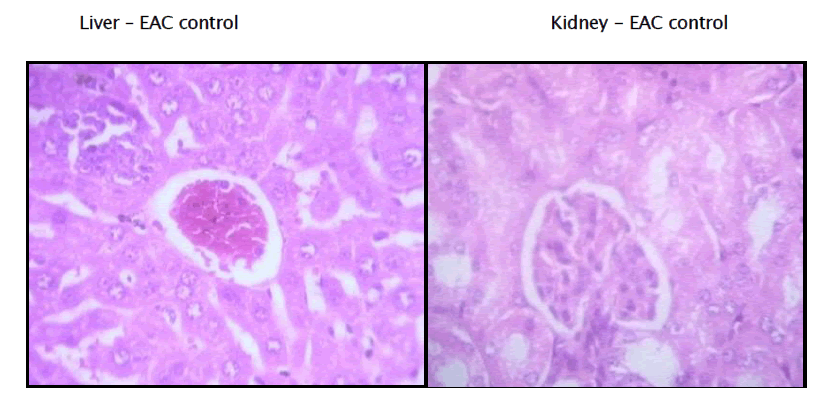
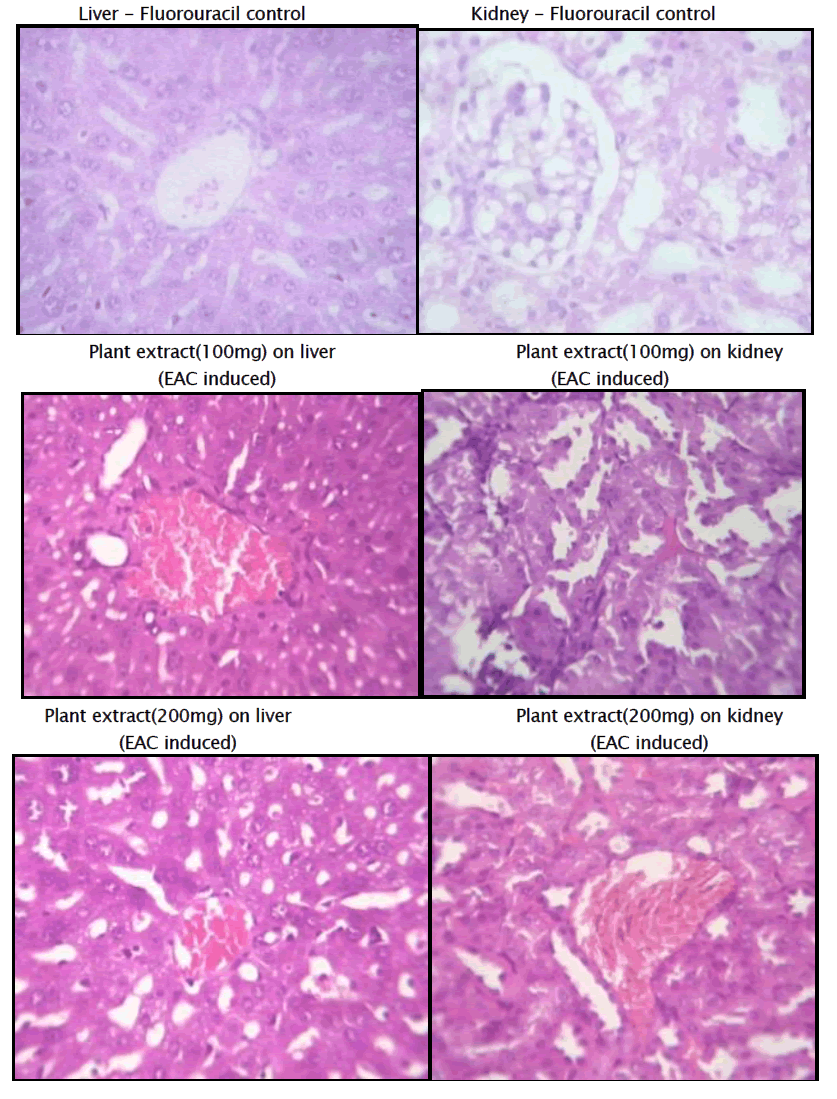
Histopathology of Liver and Kidney of EAC induced Mammary tumor
Parameter Detail discussion
The effect of flavonoid of Kigeliaafricana on the survival of tumors-bearing mice shown Table 1. The MST for the EAC control group was 17.00±0.2582 days; whereas it was 25.00±0.3651 days for Kigelia Africana (100 mg/kg/day p.o) and 31.17±0.7923 days for Kigelia Africana (200mg/kg/day i.p) treated groups. The MST 32.17±0.6009 for 5-Fluorouracil (20mg/kg/day p.o) treated group.
The ILS for Kigelia Africana (100 mg/kg/day p.o) was increased to 147.10%, for Kigelia Africana (200 mg/kg/day p.o) it was increased to 183.30% and the MST 5-Fluorouracil (20mg/kg/day p.o) was increased to 189.20% and shown in Table 2.
The effect of Kigelia Africana and 5-Fluorouracil on the body weight of tumor-bearing mice and shown in Table II. The body weight of the EAC control group was 36.37±0.2985g, whereas it was 33.75±0.2094g for Kigelia Africana (100mg) and 31.93±0.2445g for Kigelia Africana (200mg) treated groups. The body weight for 5-Fluorouracil treated group was 30.07±0.4039g.
Haematological parameters of EAC control group on Day 15 showed significant changes when compared to the treated groups. In EAC control the total WBC count was found to increase with a reduction in the haemoglobin content and the RBC count. On the other hand, flavonoid of Kigelia Africana treatment could change these altered parameters to near normal values in a dose dependent manner, and the highest dose of Kigelia Africana has produced a significant effect which is comparable to 5-Fluorouracil. The EAC control group shows degenerative changes and the kidney has vascular dilation.
The standard group receiving 5 Fluoro Uracil, The Section studied shows liver parenchyma with intact architecture. The liver parenchyma and central veins appear normal. Most of the sinusoids appear dilated and congested.
The Kidney’s architecture is intact. The Glomerulus shows normal cellularity. Tubules appear normal. Some of the blood Vessels are dilated and congested.
The 3rd group receiving 100mg of extract of Kigelia Africana Section studied shows liver parenchyma with intact architecture. Some of the sinusoids appear dilated. There are few periportal inflammatory infiltration comprising of predominantly lymphocytes. Few of the veins appear congested.
Kidney’s architecture is Intact. Glomerulus shows normal cellularity. The mesangium appear within normal limits. Tubules andInterstitium appear normal. And few blood vessels are congested.
The present experimental study showed that the extract of Kigelia Africana exhibited significant anticancer activity when compared with the standard anticancerous drug. The activity was confirmed by significant enhancement of MST, decrement of gain in average body weight and decrease of WBC count were observed. On the other hand suggesting that with further research it can also be used as adjuvant therapy in combination with the existing anticancer drugs like Fluorouracil or Methotrexate.
Based on the study we can conclude to the potential anticancer activity of flavonoid of Kigelia Africana and might be a promising anticancerous agent against breast tumor induced by Ehrlich Ascites Carcinoma. With the anti tumor activity the observations suggest that the combination has a potent anticancer activity against Ehrlich Ascites tumor. Further studies are needed to characterize the anticancer activity of the selected extract to find out the exact mechanism involved so that it can be formulated and may be tried clinically.