e-ISSN: 2322-0139 p-ISSN: 2322-0120
e-ISSN: 2322-0139 p-ISSN: 2322-0120
1Department of Neurobiology-Research, Hospital Ramón Y Cajal, Madrid, Spain
2Department of Neurology, Hospital Eugenio Espejo, Quito, Ecuador
3Foundation for Neurological Research, Madrid, Spain
4CIBERNED, Institute of Health Carlos III, Madrid, Spain
Received Date: 24/02/2017; Accepted Date: 09/03/2017; Published Date: 14/03/2017
Visit for more related articles at Research & Reviews: Journal of Pharmacology and Toxicological Studies
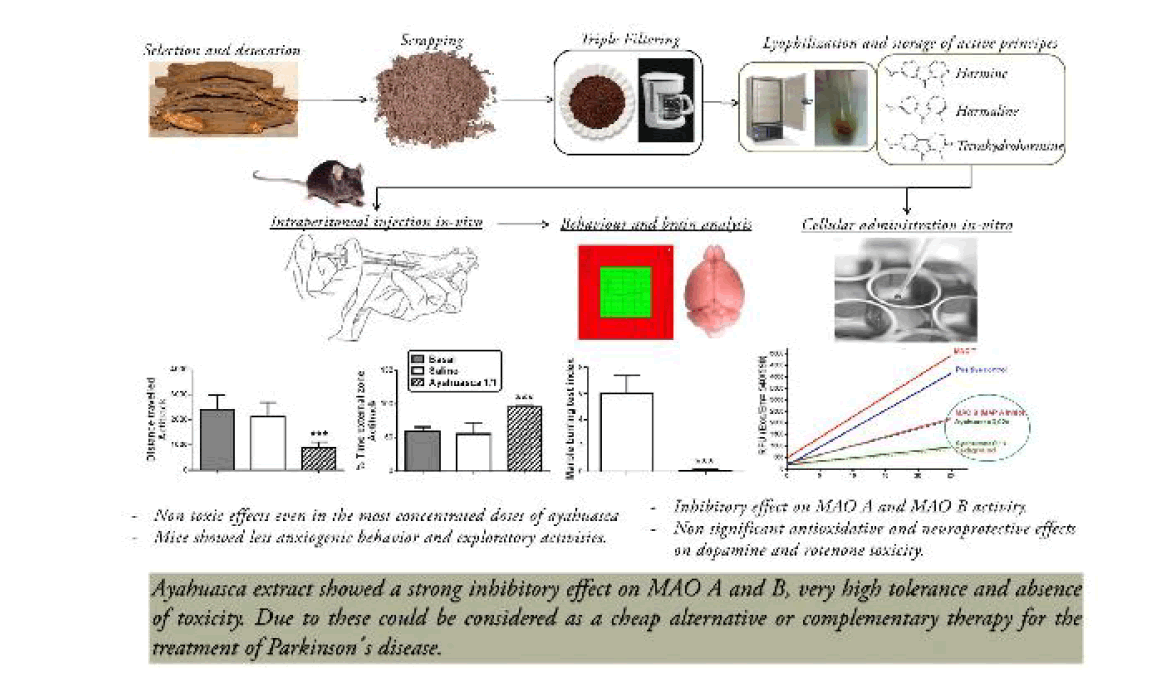
Objective: Investigate the MAO inhibitory properties, toxicity, behavioral and neuroprotective properties of ayahuasca in mice and dopamine rich neuroblastoma cells in order to assess its potential effects on PD. Methods: This study examined the effects of the soluble extract of Banisteriopsis caapi on the activity MAO in mouse brain, the MAO inhibitory activity using HPLC with electrochemical detection and the animal´s behavior in an open field and marble burying test. In vitro cell-based assays in neuroblastoma NB69 cells were employed for evaluation of the antioxidant property of ayahuasca by measuring the auto-oxidation to quinones upon dopamine exposure and its neuroprotective effects against cytotoxicity induced by DA and rotenone. The neuroprotective activity was determined by MTT, LDH and trypan blue or propidium iodide (PI) staining. Results: Intraperitoneal injection in mice of ayahuasca extract produced a significant striatal inhibitory effect on MAO A and MAO B activity. In mice striatum of ayahuasca treated mice there is an elevation of dopamine and reduction of the levels of di-hydroxy-phenyl acetic acid (DOPAC), homovanillic acid (HVA) and 5-hydroxy-indole acetic acid (5-HIAA). After ayahuasca administration, the mice display less anxiogenic behavior in marble burying test and less exploratory activities in the open field tests. Results demonstrated no significant antioxidative and neuroprotective effects of ayahuasca on dopamine and rotenone toxicity. Conclusion: Ayahuasca extract due its strong inhibitory effect on MAO A activity and more powerful inhibition of MAO B, and absence of toxicity could be used as an alternative or complementary therapy for the treatment of Parkinson´s disease.
Ayahuasca, Parkinson’s disease, Mono-amino-oxidase, Neuroprotection
Parkinson’s disease (PD) is a common neurodegenerative disorder characterized by motor (akinesia, rigidity, tremor at rest and postural abnormalities) and non-motor signs. The prevalence of PD in different European Countries is estimated from 300 to more than 600 cases/100000 habitants [1]. The cost of treatment of PD is high including medical care (medical visits, hospitalizations, medicines, physical therapy, etc.) as well as the social care (loss of jobs, early retirement, day care facilities, nursing homes, etc.). Recently, the budget for new treatments of motor complications has greatly increased. The cost of deep brain stimulation, apomorphine infusion or L-3,4 dihidroxifenilalanina.
(L-DOPA) gel intestinal administration is between 100.000 and 200.000 €/5 years [2]. This is a heavy burden for health care system of the developed countries and it is unbearable for Third World countries.
In many developing countries the drugs available for the treatment of PD are the standard formulations of L-DOPA and perhaps some dopamine agonists. However, some of these countries are rich in plants which contain products that could be candidates for the treatment of PD and other disorders. The most commonly used product is broad beans which contain substantial amounts of L-DOPA [3]. But there are other plants candidates.
Ayahuasca is a psychotropic plant tree obtained from a group of tropical Latino American plants of the genus Banisteriopsis. Up to recently ayahuasca was used in shamanic rites in Amazonian countries [4] but in the last few years its use spread to urban communities in Latino America and was brought by expanding syncretic religions to European cities [5-7].
Ayahuasca is considered to contain a unique combination of mono amino oxidase inhibitors (MAOi) and antioxidants which could be of interest for the symptomatic as well as the neuroprotective treatment of PD [8,9]. In this study we further investigated the MAO inhibitory properties of ayahuasca, its behavioral effects in rodents and its potential neuroprotective effects in dopamine rich neuroblastoma cells in vitro.
Preparation of Ayahuasca
We tried to obtain a type of infusion stable and reproducible. We obtained branches of the vine from the Amazonian part of Ecuador, from Dr. Fernando Alarcon, Hospital Eugenio Espejo, and Quito, Ecuador. In order prepare infusion with reproducible properties we selected each time three or four pieces of the branches of the plant of more or less 10-12 cm of length and around 1 cm of diameter. These branches were dried at 45ºC for 4 days and then scraped to obtain a powder. This powder was suspended in distilled water, 30.4 g of powder in 200 ml of distilled water. The suspension was brought to boiling and filtered three times and the final volume was 120 ml. This liquid was distributed in aliquots of 1.8 ml and stored in 2 ml Eppendorf tubes. The tubes are lyophilized for 14-16 h at 35ºC and kept at -80ºC.
The samples were reconstituted in 180 μl of saline. After reconstitution the samples showed 3 layers. The upper layer contained the soluble part. The middle layer was a kind of molasses. And the bottom was a black deposit. In most of the experiments we used the soluble fraction. The molasses portion was only used in one experiment in vitro concerning the toxicity of this fraction in neuroblastoma cultures. The samples used in the neuroblastoma cultures were sterilized passing them through 0.22 μm pore size membrane filters.
Animals and Treatments
Behaviors test
Nine 26 weeks old wild-type male mice (all on a C57BL/6 genetic background) grown at standard conditions of food and water, kept in our animal care department, with control of temperature and cycles of 12 h of light and darkness, of 31 to 42 g of weight were used for this experiment. The animals were injected with the soluble fraction of reconstituted ayahuasca or its dilutions 1/10, 1/100 and 1/1000, 2.75 μl/g of body weight, i.p. The control mice were injected with similar volumes of saline. These animals were used to measure the effect of ayahuasca in total activity, exploratory behavior in Actitrack LE 8811 (Panlab S.L. Barcelona, Spain) and marble burying test as previously described [10]. All animal experiments were conducted under a protocol approved by the Committee on the Ethics of Animal Experiments of the Hospital “Ramon y Cajal” (animal facilities ES280790002001), in accordance with the European Union Council Directive (86/609/EEC).
Actimeter test
Motor activity was analyzed in a computerized actimeter (Actitrack, Panlab, and Barcelona, Spain). This allows for the analysis of the distance run in the actimeter (ambulation) and the zonal distribution of the movement (anxiety and exploratory index). The exploratory index is inversely proportional to the time spent and the distance traveled in the central area away from the walls. The analysis of motor activity was done for a period of 10 min.
Marble-burying test
The mice were placed individually in plastic cages identical to their home cages, but without food or water, and with 9 marbles in 3 lines of 3 placed at equal distances between them. The animals were tested at the same time to avoid a potential confound of a within-cage order effect. A new cage, clean marbles, and fresh bedding were used for each mouse. Mice were placed in their test cages and left undisturbed for 30 min. At the end of that time, the number of marbles was recorded as: uncovered, buried completely, 2/3 covered, and half covered. This classification was used to obtain a “buried index”, as an indication of anxiolytic-like activity.
Monoamines and Metabolites and MAO Activity in Mouse Brain
Mice treated with ayahuasca or saline were sacrificed by decapitation and their brains were extracted and dissected as previously described [11]. Striatal levels of catecholamines were measured by high performance liquid chromatography with electrochemical detection (HPLC/ED) as previously described [12]. MAO activity was measured directly and indirectly. Direct measurement of global MAO activity was performed using a MAO activity kit (Biovision, Milpitas, CA, USA) according to the manufacturer’s protocol. This kit is based on the fluorometric detection of H2O2, one of the bioproducts generated during the oxidative deamination of the MAO substrate (tyramine). Changes in fluorescence were read at wavelength (Ex/Em=535/587) using an automatic microplate reader (Spectra Fluor; Tecan, Männedorf, Switzerland). Indirect measurement of MAO A and B was performed according to the ratios of 5-hydroxy-indol acetic acid/serotonin (5-HIAA/5-HT), and di-hydroxy-phenyl acetic acid/ dopamine (DOPAC/DA), respectively. Release of dopamine was measured, indirectly, according to the ratio 3-methoxy-tyramine/ dopamine (3-MT/DA).
Cell Culture Experiments
Cell culture
Human neuroblastoma NB69 cells were grown and maintained as described previously [13,14]. In brief, the cells were grown in Dulbecco's Modified Eagle's medium (DMEM) supplemented with 15% (v/v) heat inactivated fetal calf serum (FCS), penicillin (100 U/mL), streptomycin (100 mg/mL), pyruvate (1 mm) and glutamine (4 mm) (DMEM ± FCS). Cells were obtained from 4 ± 6 subcultures after thawing. Three days after plating at a density of 1 x 105 cells/mL on 35 mm diameter multi-wells, the culture medium was changed to a serum-free defined medium N2 (DMEM/Ham´s F12 1:1 supplemented with 20 nM progesterone, 100 μM putrescine, 30 nM sodium selenite and 5 μg/ml insulin which were obtained from Sigma (St. Louis, MO) and 100 μM transferrin was supplied by Boehringuer (Mannheim, Germany)). The cells were treated with dopamine 200-400 μM, rotenone 10-20 μM or vehicle in the presence and absence of ayahuasca (1/1000, 2/1000) for 24 or 48 h.
Cell viability measurements
Mitochondrial activity was analyzed with the MTT assay in NB69 cultures. MTT (0.5 mg/ml) was added to each well, and the culture plates were incubated for 2 h at 37°C. Following, the formazan salts were dissolved with 100 μl of dimethylsulphoxide (DMSO) and the absorbance (Abs) was determined at 540 nm on an automatic microliter reader (Spectra Fluor; Tecan, Männedorf, Switzerland). For necrotic cell death determination, lactate dehydrogenase activity (LDH) was performed by using a cytotoxicity detection kit in the culture medium by and trypan blue dye exclusion in cells [14-17]. In addition, cell death (apoptosis and necrosis) was measured by propidium iodide (PI) dye exclusion assay. PI positive dead cells labeled for red fluorescence, was examined using the Olympus 1×70 microscope and was counted in one-seventh of the total area of the cover slides, in predefined parallel strips using a counting reticule inserted into the microscope ocular [18].
Detection of quinones
Quinone formation, used as a rate of DA auto-oxidation, was evaluated according to the spectrophotometric measurement of the optical density at 490 nm in the culture medium [19].
Neuroprotective effects of ayahuasca in vitro
The putative neuroprotective effects of ayahuasca were tested in catecholamine rich neuroblastoma cells, NB69 cells, in vitro. The cells were cultured as previously described [14-16]. Two models of neurotoxicity in vitro were used, the treatment of the NB69 cells with dopamine and the treatment with rotenone.
The toxicity of dopamine on NB69 cells was investigated in cultures maintained in N2 defined medium after treatment with dopamine for 24 hrs. Before we studied the putative neuroprotective effects of ayahuasca in dopamine toxicity we performed a dose curve response with different concentrations of dopamine to determine the ideal dose of dopamine. We found that the minimal toxic dose of dopamine on NB69 cells in this model was 400 μM. Then the cultures were treated with saline, with dopamine, final concentration of 400 μM; ayahuasca, up to final dilutions from 0.001 to 0.02; and dopamine, 400 μM+ayahuasca from 0.001 to 0.02. The toxicity or its prevention was measured according to the levels of quinones in the medium, as an index of autoxidation; the levels of MTT, as an index of energy production; the levels of LDH, as an index of necrosis and the percentage of permeable cells according to the number propidium iodide positive cells per total cells (positive and negative cells). The effects of ayahuasca in rotenone treated NB 69 cells was investigated with similar techniques in cultures treated with saline (controls), rotenone 10 μM and 20 μM, ayahuasca 0.002 and 0.001 dilutions or with rotenone+ayahuasca at the above mentioned concentrations.
The toxicity of dopamine on NB69 cells was investigated in cultures maintained in N2 defined medium after treatment with dopamine for 24 h. Before we studied the putative neuroprotective effects of ayahuasca in dopamine toxicity we performed a dose curve response with different concentrations of dopamine to determine the ideal dose of dopamine. We found that the minimal toxic dose of dopamine on NB69 cells in this model was 400 μM. Then the cultures were treated with saline, with dopamine, final concentration of 400 μM; ayahuasca, up to final dilutions from 0,001 to 0,02 and dopamine, 400 μM+ayahuasca from 0.001 to 0.02. The toxicity or its prevention was measured according to the levels of quinones in the medium, as an index of autoxidation; the levels of MTT, as an index of energy production; the levels of LDH, as an index of necrosis and the percentage of permeable cells according to the number propidium iodide positive cells per total cells (positive and negative cells). The effects of ayahuasca in rotenone treated NB 69 cells was investigated with similar techniques in cultures treated with saline (controls), rotenone 10 μM and 20 μM, ayahuasca 0.002 and 0.001 dilutions, or with rotenone+ayahuasca at the above mentioned concentrations.
The statistical analysis was performed with assistance of the Graph Pad Prism package, version 5.01, from Graph Pad Software Inc., 7825 Fay Av., La Jolla, CA, USA. Experiments performed with two experimental groups were analyzed with student’s t test. Experiments performed with multiple experimental groups were analyzed with one way analysis of variance, followed by the Newman-Keuls test for multiple comparisons. Differences were considered significant when p<0.05.
Motor Behavior of the Mice Treated with Ayahuasca
The animals treated with 2.75 μl/g of body weight, i.p. of ayahuasca, diluted 1/100 and 1/1000, did not show abnormal behavior or neurological deficits in open field, social behavior or in Actitrack. Animals treated with ayahuasca undiluted presented a reduction of global motor activity (Figure 1a) as well as an increased percentage of time spent in the external part of the Actitrack (Figure 1b). With regard to the marble burying behavior it was reduced in mice treated with ayahuasca after injection of the tea at the dilution of 1/10 or undiluted (Figure 1c). These findings are consistent with a decreased exploratory behavior and with a reduction of anxiety.
Effects of ayahuasca on monoamines, metabolites and MAO activity in striatum
Adding ayahuasca undiluted, 5 μl, to the enzymatic cocktail (final ayahuasca concentration 1/10), reduced the MAO activity to 28,5% of the total MAO activity in the striatum (Figures 2a and 2b). The levels of dopamine (DA) were almost doubled in striatum of animals treated with ayahuasca (Figure 3a) while the levels of norepinephrine (NE) and serotonin (5-HT) were unchanged (Figures 3b and 3c). With regard to monoamine metabolites the levels of di-hydroxy-phenyl acetic acid (DOPAC), homovanillic acid (HVA) and 5-hydroxy-indole acetic acid were greatly reduced (Figures 3d-3f) while those of 3-methoxy-tyramine were increased (Figure 3g). These changes, all together, signify that ayahuasca is a potent inhibitor in vitro of both MAO A and MAO B. The effect is more potent on MAO B than on MAO A since the ratio DOPAC/DA (Figure 3h) is smaller than the ratio 5-HIAA/5-HT (Figure 3i). The levels of 3-methoxy tyramine (3-MT) were increased in ayahuasca treated animals (Figure 3g) suggesting that ayahuasca increases the release of DA and its extracellular metabolism via catechol-ortho-methyl transferase (COMT).
Figure 3: Ayahuasca modulate monoamine, metabolites and MAO activity in WT mice. A) DA levels, B) NA levels, C) 5-HT levels. D-G) monoamine metabolites (DOPAC, HVA, 5-HIAA, 3MT). H) MAO-B activity. I) MAO-A activity. Results are expressed in nanograms per gram of fresh tissue. Values are expressed as the mean ± SEM (n=4-5) animals in each experimental group). Statistical analysis was performed by Student t test. *p<0.05, **p<0.01, ***p<0.001 WT mice treated with Ayahuasca versus WT mice treated with vehicle.
Effects of Ayahuasca on Cell Viability and Resistance to Neurotoxins
The treatment with ayahuasca, upper layer or middle layer, of the NB69 cultures, at dilutions form 0.01 to 0.1, produced a dose dependent reduction of cellular viability as shown by the MTT levels (Figures 4a and 4b). Likewise, treatment of the cultures with ayahuasca obtained from both the upper or the middle layer, at dilutions from 0.01 to 0.1, increased the rate of necrosis as shown by the levels of LDH (Figures 4c and 4d).
Figure 4: Dose-dependent ayahuasca neurotoxic effects on cell viability in NB69 cells. After 6 days in vitro, the cells were treated with ayahuasca (0.001x-0.1x) for 24 h. A, B) MTT activity of soluble and molasses fractions respectively. C and D) LDH activity. Values are the mean ± SEM from two independent experiments with 6 replicates each. Statistical analysis was performed by Student t test. ***p<0.001 versus controls cultures treated with vehicle.
In the model of dopamine toxicity to NB69 cells there is an increase in the levels of quinones levels and in the percentage of propidium iodide (IP) positive cells (Figures 5a-5c). Co-treatment of these cells with dopamine, 200 μM+ayahuasca, 1/1000 or 2/1000, does not change the levels of quinones and it even increases the percentage of propidium iodide increased by dopamine. In NB69 cells treated with dopamine, 400 μM, the findings are the same but in addition there is a reduction of MTT levels, which is not reverted by co-treatment with ayahuasca and that is even further increased with the ayahuasca dilution of 2/1000 (Figure 6).
Figure 5: Effects of dopamine and ayahuasca on cell death and quinone production in NB69 cells. After 5 days in vitro (DIV), the cells were pre-treated with Ayahuasca 15 min before DA 200 μM for 24 h in defined medium (DM) without serum. A) Propidium iodide dye images (scale bar=30 μm). B) Effect of 200 μM DA, and ayahuasca treatment on quinone production, at 490 nm. C) Percentage of cell death, expressed as the ratio of propidium iodide cells/total cells. Values are the mean ± SEM from two independent experiments with 6 replicates each. Statistical analysis was performed by one-way ANOVA followed by Newman Keuls multiple comparison test. ***p<0.001 vs. control group; +++p<0.001 ayahuasca+DA vs. DA-treated cultures.
Figure 6: Effects of dopamine and ayahuasca on cell viability and quinone production in NB69 cells. After 4 days in vitro (DIV), the cells were pre-treated with Ayahuasca 15 min before DA 400 μM for 48 h in defined medium (DM) without serum. A) Photomicrograph showing trypan blue dye exclusion (scale bar=30 μm). B) Percentage of necrotic cells. C) Mitochondrial activity [3-(4,5-dimethylthiazol-2-yl)-2,5 biphenyl tetrazolium bromide (MTT) assay]. D) Effect of 400 μM DA, and ayahuasca treatment for 48 h on quinone production, at 490 nm. Values are the mean ± SEM from two independent experiments with 6 replicates each. Statistical analysis was performed by one-way ANOVA followed by Newman Keuls multiple comparison test. ***p<0.001 vs. control group.
In the model of rotenone toxicity for NB69 cells we found that rotenone reduces the levels of MTT at concentrations of 20 μM and increases the levels of LDH from 5 to 20 μM (Figures 7a and 7b). Rotenone reduces the number of NB69 cells and this effect is not reversed by ayahuasca (Figures 7c and 7d).
Figure 7: Effects of rotenone and ayahuaska on on cell viability in NB69 cells. A and B) Dose–response curve of the effects of Rotenone on NB69. A) MTT levels of rotenone from 0 to 20μM and B) LDH activity. C) Phase contrast photomicrographs of NB69 cultures pre- treated with ayahuasca 15 min before rotenone 20 μM for 24 h (scale bar=50 μm). The cells were cultured in defined medium (DM) without serum (scale bar=50 μm). D and E) Mitochondrial MTT activity in NB69 cells treated with rotenone 10 and 20 μM for 24 h. Values are the mean ± SEM from two independent experiments with 6 replicates each. Statistical analysis was performed by one-way ANOVA followed by Newman Keuls multiple comparison test. *p<0.05***, p<0.001 vs. control group.
We have investigated the effects of ayahuasca in mice and in cellular and tissue systems rich in catecholamines. Our most important findings are that ayahuasca is well tolerated by mice; it does not produce significant side effects, in mildly decreases exploratory behavior and reduces anxiety. In mice striatum of ayahuasca treated mice there is an elevation of dopamine and 3-MT, and reduction of the levels of DOPAC, HVA and 5-HIAA. These changes suggest that ayahuasca has a strong inhibitory effect on MAO A activity and a more powerful inhibition of MAO B. The elevation of the levels of 3-MT is difficult to interpret in the presence of MAO B inhibition but it suggests an enhancement of the release of dopamine by ayahuasca, an effect which has already been reported [20].
Ayahuasca is a type of herbal tea obtained from different types of Amazonian plants of the genus Banisteriopsis, most frequently Banisteriopsis Cape. This plant contains several compounds with strong MAO inhibition properties, mainly harmine, and smaller concentrations of harmaline and harmalol. These substances combine the MAO inhibition properties with strong antioxidant effects [8] and frequently are mixed with other plant extracts rich in di-methyl-tryptamine, a plant hallucinogen which acts in serotonin receptors.
Ayahuasca is a ritual beverage traditionally used in shamanic ceremonies. Recently, due to its good tolerance, its use has spread to urban communities in Latino America, syncretic religious groups in America and Europe, and travelers from all over the world. Ayahuasca is usually relatively well tolerated but there are cases of deaths reported [21]. The great majority of its consumers present vomits from 30-40 min to several hours after its ingestion and times diarrhea, sweating, tachycardia and changes in the blood pressure. This period is followed by an impression of opening of the mind, with increased perception and sensation; and then it comes a period of calm, peace and tranquility.
We have investigated the potential use of ayahuasca in patients with PD. This potential use is based in two rationales: 1) To provide cheap and effective therapeutic plant extracts in countries that could not afford the prizes of recently marketed medicines, and 2) To look for anti-parkinsonian agents with therapeutic properties. The first point will be clear if one takes in consideration than many Latino American and other Third World countries have limited treatments for PD. The potential neuroprotective properties of ayahuasca may be derived from its MAO B inhibitory activity which may reduce the levels of free radicals. In addition, the ingestion of ayahuasca increases the serotonergic neurotransmission and it has been claimed that the stimulation of 5-HT 1A receptors of astrocytes improves the survival of nigral neurons [22].
Regretfully, we could not find neuroprotective effects in the two models of toxicity on NB69 cells that we tested the treatment with high concentrations of dopamine or with rotenone. Toxicity of dopamine in dopamine neurons is thought to be caused by several mechanisms including autoxidation of dopamine to quinones and excessive free radical production due to the enhanced metabolism of dopamine via MAO B. We found in our study that ayahuasca strongly inhibits MAO B activity but it does not suppress Quinone formation. Rotenone toxicity is due to inhibition of complex I of the mitochondrial respiratory chain and therefore, to deprivation of energy. Ayahuasca could prevent that if it produces stimulation of the 5-HT 1A since these receptors have been linked with neuroprotection in cerebral ischemia and mitochondrial failure [23-25]. Since the results of the neuroprotection studies were negative the potential effects of ayahuasca should be tested in other models of PD.
Juan Perucho and Maria Jose Casarejos participated in all experimental work; Justo García de Yebenes, Maria Angeles Mena and Fernando Alarcon applied for grants, designed the experimental protocols and prepared the manuscript.
The authors declare that they have no competing, financial or non-financial, interests.
This study has been supported in part by grants from the Spanish Ministry of Health, FIS 2010/172, CIBERNED PI 2010/06 and CAM 2011/BMD-2308. The authors thank Ms Ana Gomez and Mrs Maria Paz Munoz for excellent technical assistance.