Pharmacology, Phytochemistry and Safety of Aphrodisiac Medicinal Plants: A Review
Poonam Sharma1, Priyanka Bhardwaj2, Tasleem Arif2, Imran Khan1 and Rambir Singh2*
1Department of Zoology, Bundelkhand University, Jhansi, Uttar Pradesh, India.
2Department of Biomedical Sciences, Bundelkhand University, Jhansi, Uttar Pradesh, India.
- *Corresponding Author:
- Rambir Singh
Department of Biomedical Sciences, Bundelkhand University, Jhansi, Uttar Pradesh, India
Mobile:+91 9473583251
Fax: +91 510-2320761
Received date: 23 March 2014 Revised Date: 27 April 2014;Accepted date: 29 April 2014
Visit for more related articles at Research & Reviews: Journal of Pharmacology and Toxicological Studies
Abstract
The history of sexual medicine and management of male sexual dysfunction (MSD) is as old as human civilization. The modern life styles and environmental conditions have increased prevalence of MSD with age. To address this problem a number of therapeutic strategies including the use of medicinal plants have been advocated for management of MSD. Large numbers of research papers regarding aphrodisiac activity of medicinal plants have been published in past few years. This review compiles data on the potential aphrodisiac activity of medicinal plants possessing effective dose of less than equal to 200 mg/kgbw or equivalent. The toxicity studies and phytochemical data available for the active extract or active plant part have also been incorporated in this review. Data regarding plant part, dose, animal model, compounds isolated and mechanism of aphrodiasic activity was tabulated. Medicinal plants possess an untapped source of aphrodisiac molecules. The review identified that Bryonia laciniosa, Caesalpinia benthamiana, Ferula harmonis, Montanoa tementosa, Syzygium aromaticum, Turnera aphrodisiaca, Spilanthes acmella, Turnera aphrodisiaca, Turnera diffusa, and Tribulus terrestris plants possess potential aphrodisiac activity. The safety in long term usage and low cost may be added advantage associated with use of herbal aphrodisiacs.
Keywords
Plant aphrodisiacs, Safety index, Phytochemical analysis, Aphrodisiac activity.
Introduction
Sexual health is a state of complete physical, mental and social well being in all aspects related to the reproductive system. Compromised sexual abilities may lead to infertility. Male sexual dysfunction (MSD) resulting in unsuccessful intercourse may adversely affect the personal and social life of the suffer couples and also contributes to infertility. MSD may be due to decreased libido, erectile dysfunction and disorders of ejaculation. A number of factors including psychological disturbances (performance anxiety, strained relationship, depression, stress, guilt and fear of sexual failure), deficiencies in sex hormones (testosterone deficiency), chronic diseases (diabetes, hypertension, atherosclerosis, venous leakage), neurological disorders (Parkinson’s disease, Alzhemier’s disease, spinal cord or nerve injury), side effects associated with chronic use of drugs (anti-hypertensives, central agents, psychiatric medications, antiulcer, antidepressants, anti-androgens), life style related complications (chronic alcohol abuse, cigarette smoking) and aging are known to contribute to MSD [1,2].
A human male may suffer from MSD at any stage of life but its risk increases with age. A population based study in US revealed that prevalence of MSD was 12 percent in those younger than 59 years, 22 percent in those 60 to 69 years of age, and 30 percent in those older than 69 years [3]. As per an estimate over 320 million people in the Westernized nations will be develop MSD by 2025 [4]. The current epidemiological data suggests that MSD needs immediate medical intervention and newer therapeutic strategies are required for its management. A number of treatment options are available for management of MSD. These options includes psychological and behavioral therapy, non surgical treatments using constructive rings and vacuum pumps, surgical treatment such as penile prosthesis, penile implants and venous ligation, hormone replacement therapy and intervention of chemotherapeutic agents [5]. The chemotherapeutic agents used for treatment of MSD are known as ‘aphrodisiac’.
Discovery of oral phosphodiesterase type 5 (PDE5) inhibitors particularly sildenafil (Viagra), tadalafil (Cialis), and vardenafil (Levitra) has revolutionized treatment of MSD [6]. Sidenafil citrate is the most prescribed PDE5, recommend in almost more than 70% of patients suffering from MSD. Mild to moderate headache, facial flushing, nasal congestion and dyspepsia are the most common adverse effects of PDE5 treatment [7]. Severe effects on PDE5 treatment have been reported in patients suffering from hypertension, hence careful clinical examination is a must before prescribing PDE5.
Aphrodisiac medicinal plants
The side effects associated with these synthetic drugs necessitated search for safer and effective aphrodisiac agents especially of herbal origin. Medicinal plants represent an extraordinary reservoir of active ingredients [8,9]. Aphrodisiac activity of medicinal plants from a number of medicinal systems especially Ayurvedic [10] and Traditional Chinese medicinal has been reported [11]. Yohimbine, an indole alkaloid extracted from the bark of West African yohim trees was the first natural aphrodisiac molecules introduced for management of MSD. Several clinical trials reported various efficacy rates of yohimbine ranging from 34% to 73% [12] compared to Viagra. Approval of yohimbine by Food and Drug Administration, USA for clinical use, further propelled use of plant based aphrodisiac agents and intensified research in this area. The plant based aphrodisiac agents are relatively low in cost and safe as compared to synthetic PDE5.
Safety issue associated with aphrodisiac medicinal plants
Plants are extensively used to manage MSD. A number of research papers including some reviews have been published recently on the aphrodisiac activity of medicinal plants [8]. Although a number of plants with potential aphrodisiac activity have been identified through these reviews, the safety issue associated with the active extracts of these plants needs attention. The safety of plant based medicine needs to be evaluated essentially before recommending for human consumption. So, considering the merits of plant based aphrodisiac agents, an attempt has been made to review data on aphrodisiac activity and safety. We also tried to incorporate the data on phytochemicals, purified either from the active extracts or the plant part exhibiting aphrodisiac activity.
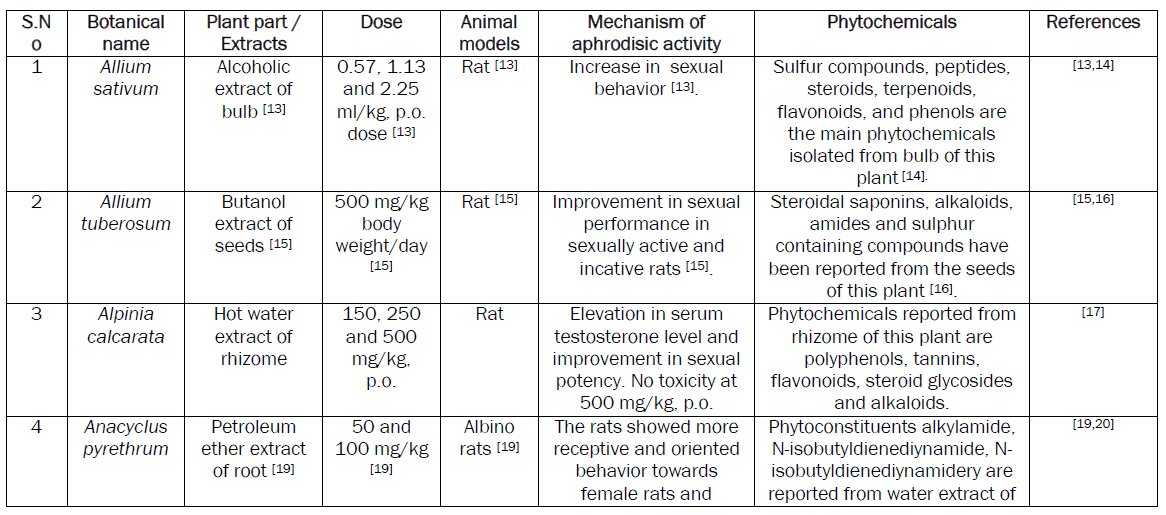
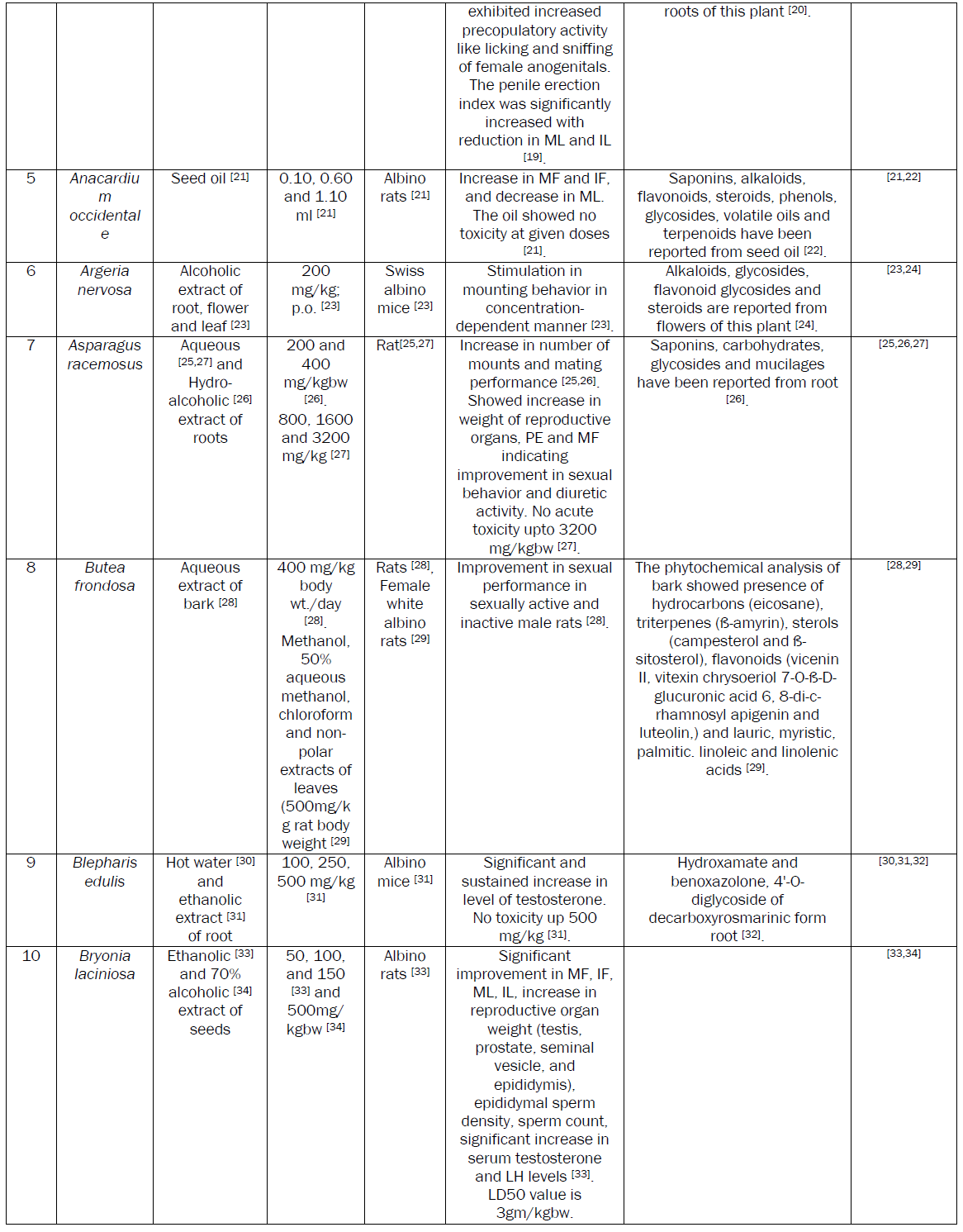
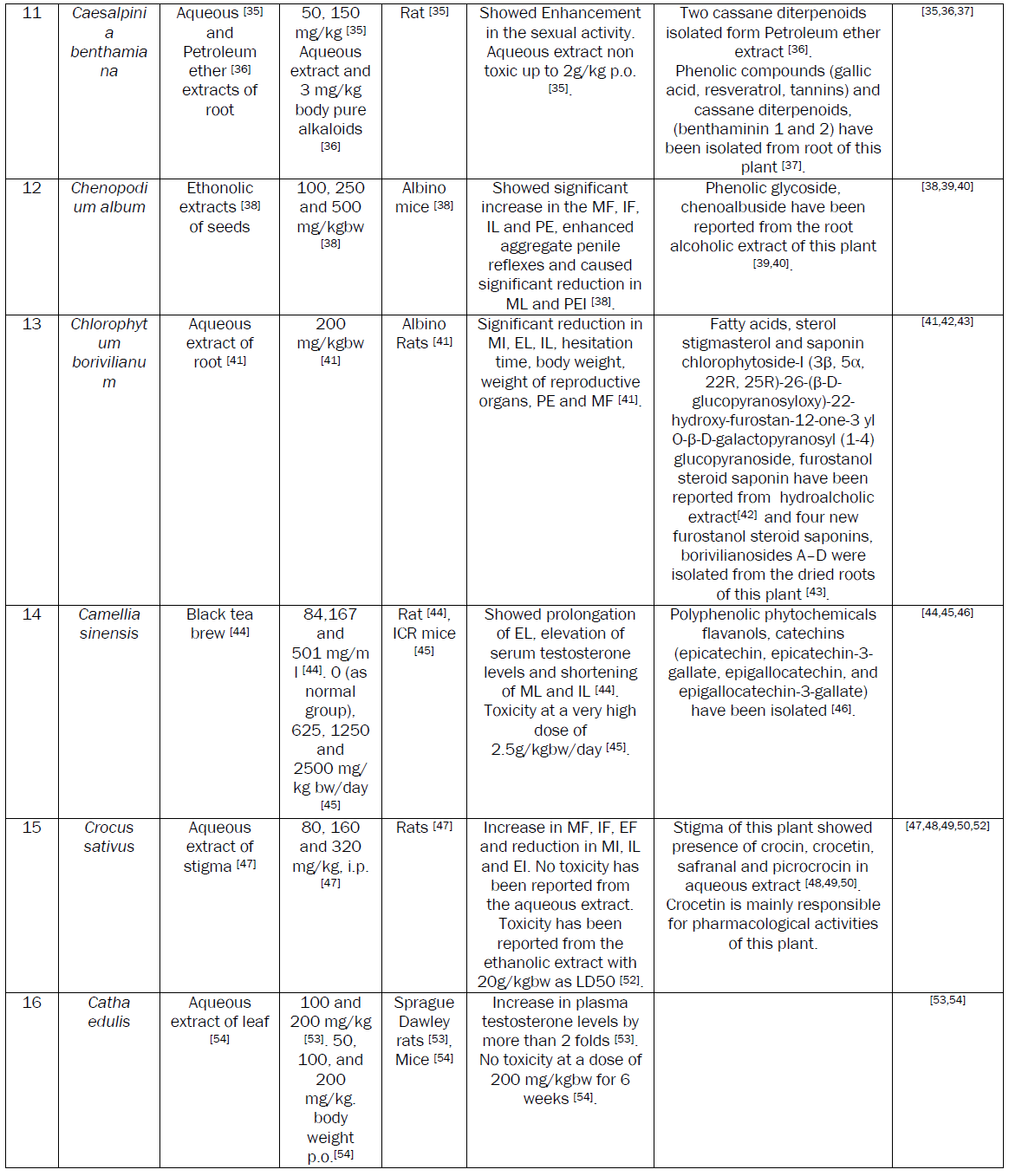
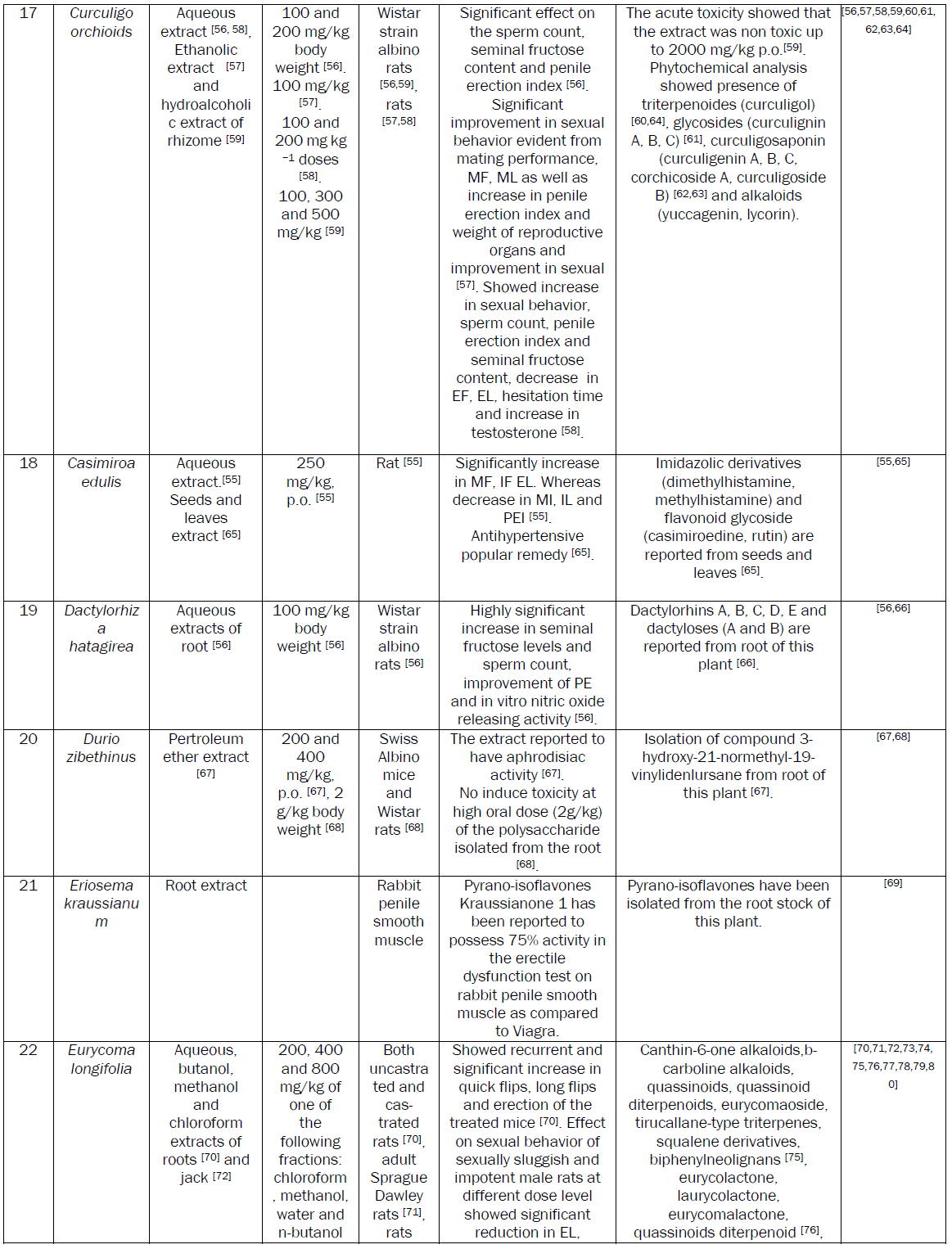
Conclusion
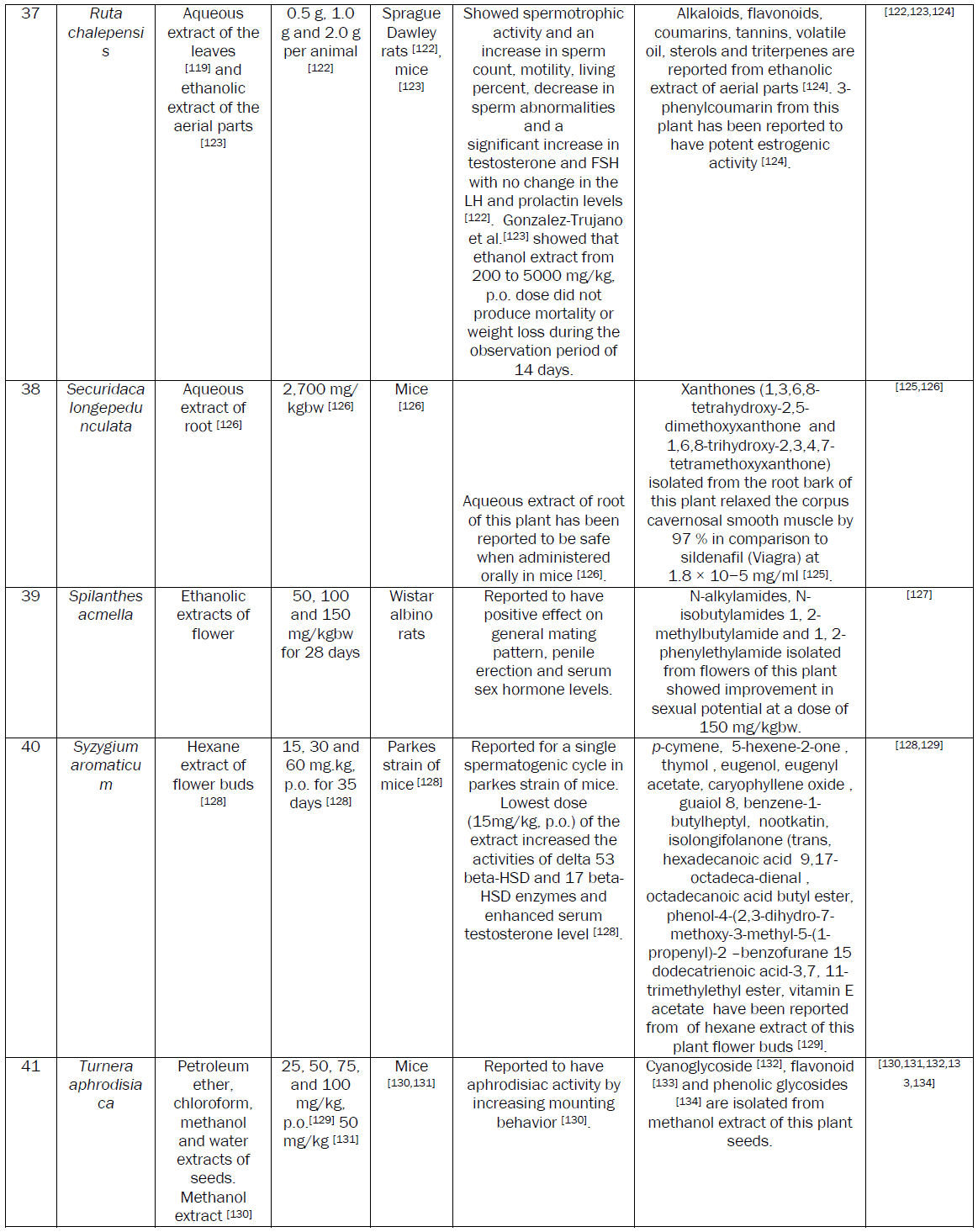
The demand for herbal drugs has increased in developed as well as developing countries because of their good aphrodisiac activity and safety. The review identified that Bryonia laciniosa, Caesalpinia benthamiana, Chlorophytum borivilianum, Ferula harmonis, Montanoa tementosa, Mucuna pruriens,Syzygium aromaticum, Turnera aphrodisiaca, Spilanthes acmella, Turnera aphrodisiaca, Turnera diffusa, ,Tribulus terrestris, Turnera aphrodisiaca and Withania somnifera plants possess potential aphrodisiac activity. The ED50 of active extracts of these plants have been reported to be less than equal to 50mg/kgbw. Two potential aphrodisiac compounds namely 1,3,6,8-tetrahydroxy-2,5-dimethoxyxanthone and 1,6,8-trihydroxy-2,3,4,7-tetramethoxyxanthone from Securidaca longepedunculata relaxed the corpus cavernosal smooth muscle by 97 % as comparison to sildenafil where as kraussianone 1 from Eriosema kraussianum relaxed rabbit penile smooth muscles by 75% as compared sildenafil. These purified phytochemicals may be picked up for large scale clinical trials in drug discovery programme. No toxicity has been reported at effective dose of the extract possessing aphorodisc activity in the above mentioned plants. In safety studies, the LD50 of some of the plants was much higher as compared to ED50. The reported LD50 is 20g/kgbw for Crocus sativus [52], 2g/kgbw for Pedalium murex [109] and 2.5g/kgbw for Camellia sinensis [45].
Mechanism of aphrodisiac activity of medicinal plants
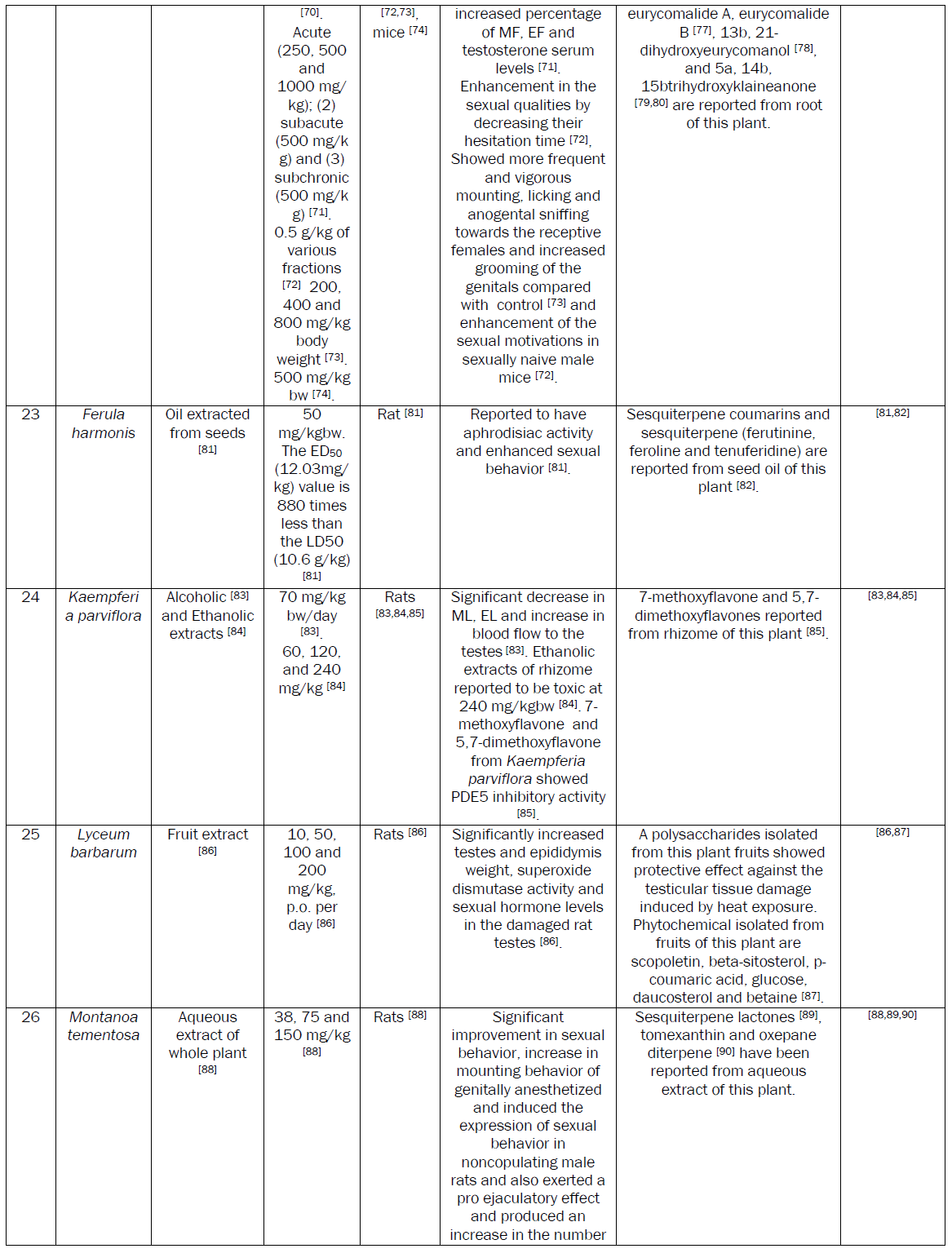
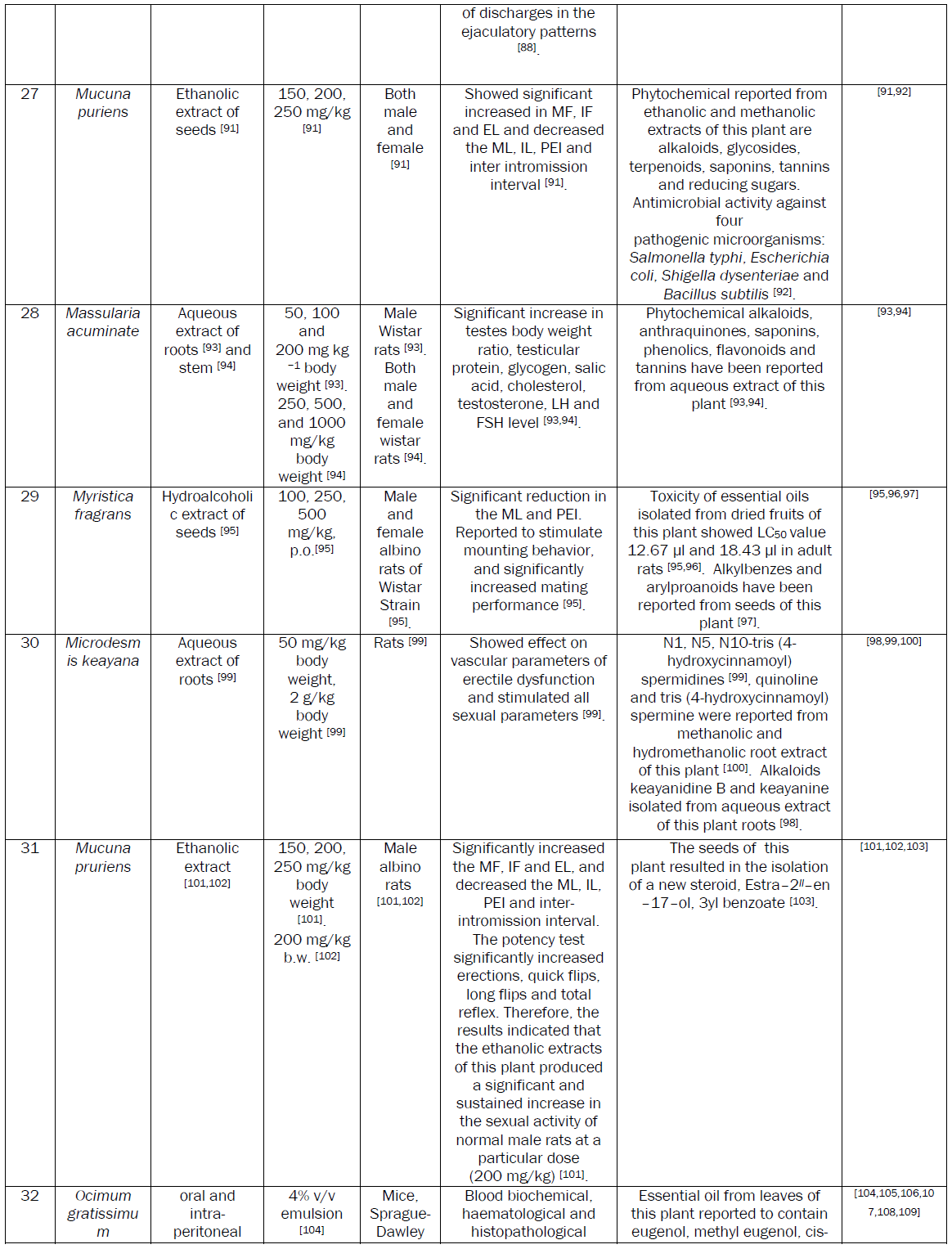
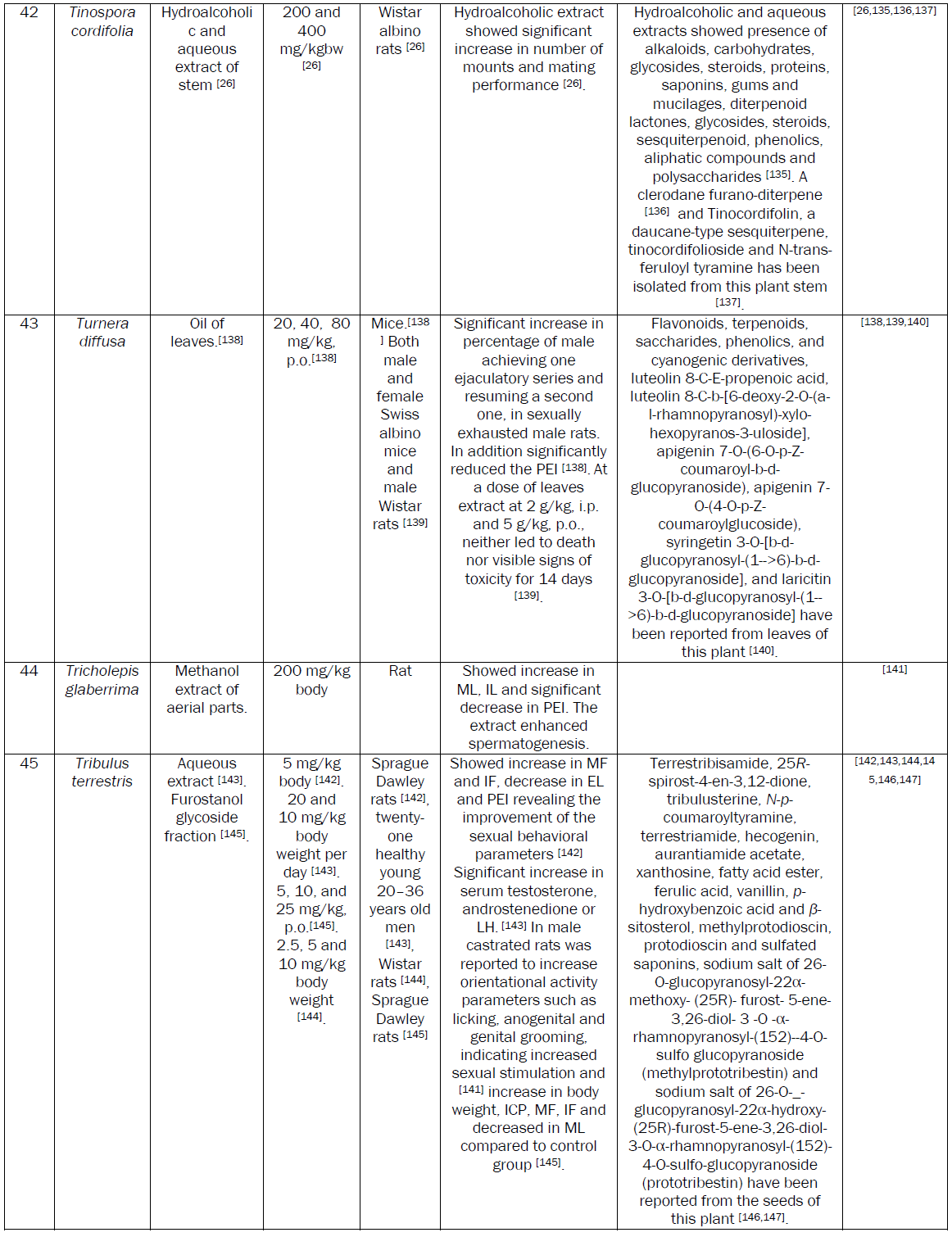
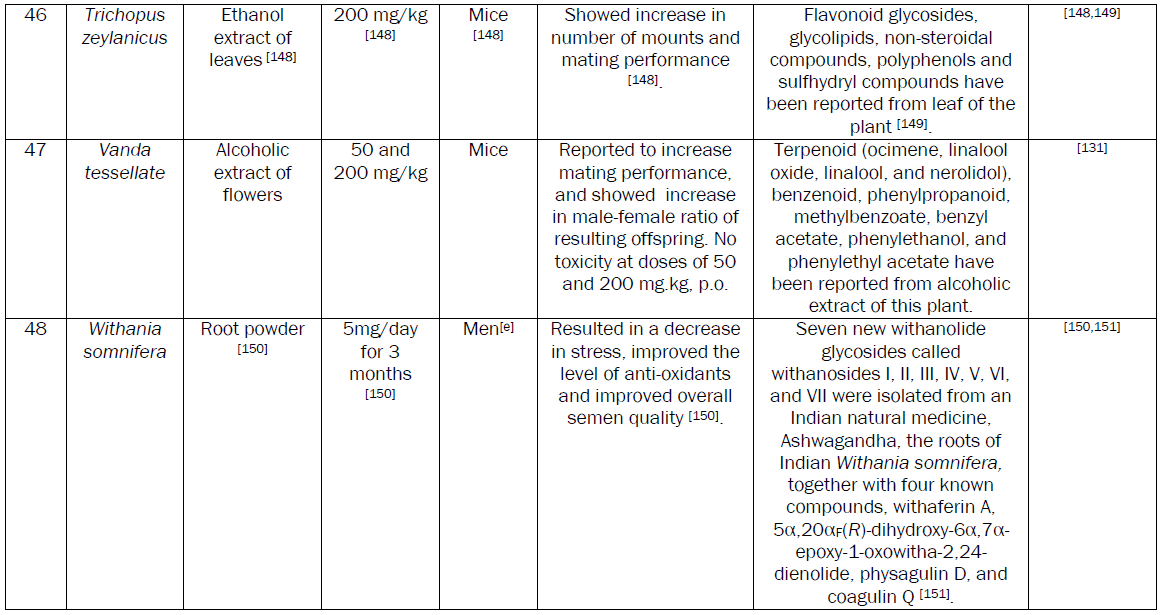
Increase in serum testosterone level is the chief mechanism of aphrodisiac action shown by a number of medicinal plants. Ethanolic extract of Blepharis edulis roots [31], Camellia sinensis [44], Aqueous extracts of Massularia acuminate roots [93], Ruta chalepensis leaves [119], Tribulus terrestris fruits [143] exhibited aphrodicisc activity by enhancing testosterone level. Aqueous extract of Massularia acuminate roots [93] and Ruta chalepensis leaves [119] also enhanced FSH and LH along with testosterone. 7-methoxyflavone and 5,7-dimethoxyflavone from Kaempferia parviflora showed PDE5 inhibitory activity [85].
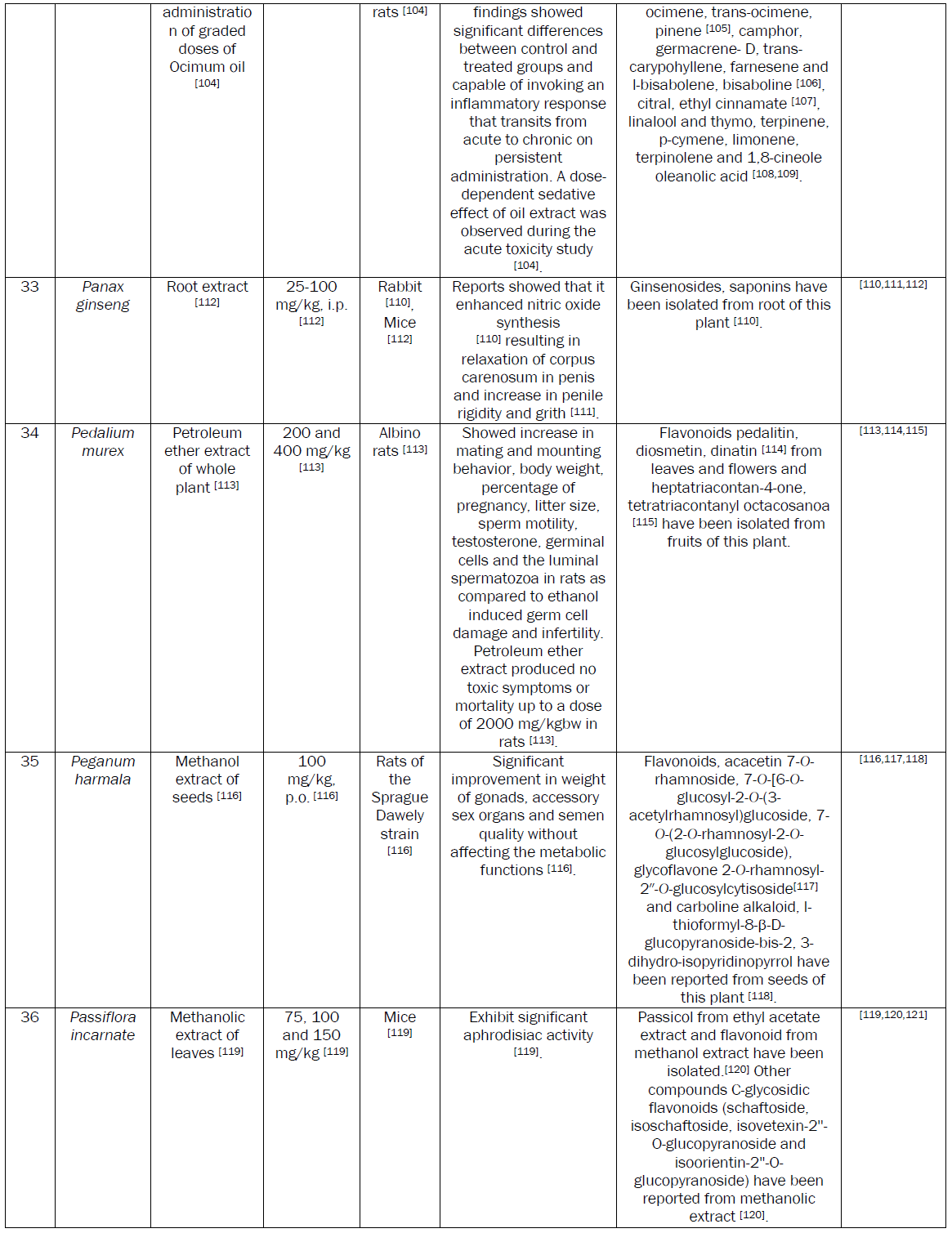
Panax ginseng showed aphrodiasic activity by nitric oxide linked mechanisms. Reports showed that it enhanced nitric oxide synthesis [107,108] resulting in relaxation of corpus carenosum in penis and increase in penile rigidity and grith.
It is concluded that medicinal plants possess an untapped source of aphrodisiac molecules. The safety and low cost may be added advantage associated with use of herbal aphrodisiacs.
Abbreviations
Mount frequency (MF), Intromission frequency (IF), Mount latency (ML),Intromission latency (IL), Ejaculation latency (EL), Ejaculation frequency (EF), intracavermous pressure (ICP), Post ejaculatory interval (PEI), Mount latencies (ML), Intromission latencies (IL), Ejaculation latencies (EL), The introduction of one organ or part into another (IF), The time interval between the introduction of the female and the first mount by the male (ML), The time interval from the time of introduction of the female to the first intromission by the male (IL), The time interval between the first intromission and ejaculation and ejaculation frequency (EL), Penile erection (PE), Luteinizing hormone (LH) and Follicle stimulating hormone (FSH).
References
- Guay T, Spark RF, Bansal S, Cunningham GR, Goodman NF, Nankin HR, et al. American Association of Clinical Endocrinologist: Medical Guidelines for Clinical Practice for the evaluation and treatment of male sexual dysfunction A couple’s problem. EndocrinolPract. 2003;9:78-95.
- Kandeel FR, Koussa VKT, Swerdloff RS. Male sexual function and its disorders: Physiology, Pathophysiology, Clinical Investigation and Treatment. Endocr Rev. 2001;22:342-388.
- Bacon CG, Mittleman MA, Kawachi I, Giovannucci E, Glasser DB, Rimm EB. Sexual function in men older than 50 years of age: results from the health professionals follow-up study. Ann Intern Med. 2003;139:161-168.
- McKinlay JB. (2000). The worldwide prevalence and epidemiology of respective PDEs, could achieve greater enhance-erectile dysfunction. Int J Impot Res. Suppl 4.
- Grenier RF, Byers ES. Rapid ejaculation: a review of conceptual, etiological and treatment issues. Arch Sex Behav. 1995;22:447-472.
- Vitezic D, Pelcic JM. Erectile dysfunction: oral pharmacotherapy options. Int J ClinPharmacolTher. 2002;40:393-403.
- Gresser U, Gleiter CH. Erectile dysfunction: comparison of efficacy and side effects of the PDE-5 inhibitors sildenafil, vardenafil and tadalafil – review of the literature. Eur J Med Res. 2002;7:435-46.
- Shamloul R. Natural Aphrodisiacs. J Sex Med. 2010;7:39-49.
- Sandroni P. Aphrodisiacs past and present: a histrocial review. ClinAuton Res. 2001;11:3003-3007.
- Acharya VYT. CharakaSamhuita with AyurvediaDipika commentary of chakaoanidatta. 5th edVaransi: Chaukhama Sanskrit Samsthan. 2001;301-350.
- Jia L, Zhao Y, Liang XJ. Current evaluation of the millennium phytomedicine- ginseng (II): Collected chemical entities, modern pharmacology, and clinical applications emanated from traditional Chinese medicine. Curr Med Chem. 2009;16:2924-42.
- Klinger T, Noe S, Riischke J, Muller S, O Benkeir. Effects of yohimbine on sexual experiences and nocturnal penile tumescence and rigidity in erectile dysfunction. Arch Sex Behav. 1996;25:1-10.
- Mullaicharam AR, Karthikeyan B, Umamaheswari R. Aphrodisiac property of Allium sativum Linn extract in male rat. HamdardMedicus. 2004;47:30-35.
- Agarwal K. Therapeutic actions of garlic constituents. Med Res Rev. 1996; 16:111-124.
- Guohuaa H, Yanhuab L, Renganga M, Dongzhib W, Zhengzhia M, Huaa Z. Aphrodisiac properties of Allium tuberosum seeds extract. J Ethnophamacol. 2009;122:579-582.
- Hostettmann, K, Marston A, Wolfender JL. (1995). Strategy in the search for new biologically active plant constitutents, in: Hostettmann K, Marston A, Maillard M, Hamburger M, eds, Phytochemistry of Plants Used in Traditional Medicine. Proceedings of the Phytochemical Society of Europe Oxford, Oxford Science Publications.pp.18-45.
- Ratnasooriya R, Jayakody JR. Effects of aqueous extract of Alpiniacalcarata rhizomes on reproductive competence of male rats. ActaBiol Hung. 2006;57:23-35.
- Arambewela LSR, Arawwawala LDAM. Standardization of Alpiniacalcarata Roscoe rhizomes. Pharmacog Res. 2010;2:285-288.
- Sharma V, Thakur M, Chauhan NS, Dixit VK. Evaluation of the anabolic, aphrodisiac and reproductive activity of Anacyclus pyrethrum DC in male rats. Sci Pharm. 2009;77:97-110.
- Bendjeddou D, Lalaoui K, Satta D. Immunostimulating activity of the hot water- soluble polysaccharide extracts of Anacyclus pyrethrum, Alpinia galangal and Citrulluscolocynthis. J Ethnopharmacol. 2003;88:155-160.
- Mbatchou VC, Kosoono I. Aphrodisiac activity of oils from Anacardiumoccidentale L seeds and seed shells. Phytopharmacol. 2012;2:81-91.
- Kannan VR, Sumathi CS, Balasubramanian V, Ramesh N. Elementary Chemical Profiling and Antifungal Properties of Cashew (Anacardiumoccidentale L.) Nuts. Botany Research International. 2009;2(4):253-257
- Subramoniam A, Madhavachandran V, Ravi K, Anuja VS. Aphrodisiac property of the elephant creeper Argyreia nervosa. J EndocrinolReprod. 2007;2:82-85.
- Ashish J, Modi SS, Khadabadi UA, Deokate IA, Farooqui SL, Deore S, et al. Argyreiaspeciosa Linn Phytochemistry, pharmacognosy and pharmacological studies. J Pharmacol and Phytoth. 2010;2:34-42.
- Thakur M, Bhargava S, Dixi VK. Effect of Asparagus racemosus on sexual dysfunction in hyperglycemic male rats. Pharm Biol. 2009;47:390-395.
- Wani, JA, Rajeshwara N, Achur RK, Nema RA. Phytochemical Screening and Aphrodisiac Property of Tinosporacordifoli. Int J PharmaClin Res. 2011;3:21-26.
- Kumar MC, Udupa AL, Sammodavardhana K, Rathnakar UP, Shvetha U, Kodancha GP. Acute toxicity and diuretic studies of the roots of Asparagus racemosusWilld in rats. West Ind Med J. 2010;59:3-6.
- Ramachandran S, Sridhar Y, Kishore G, Sam S, Saravanan M, Thomas LJ, et al. Aphrodisiac activity of ButeafrondosaKoen ex Roxb extract in male rats. Phytomed. 2004;11:165-168.
- Hefnawy MS, Mohamed DA, Khamis NE, Afifi AH, Mabry TJ. Phytochemical and biological studies of Buteafrondosaroxb. Leaves growing in Egypt. Pharmacognosia. 1984;22:201-210.
- SY, Tsai HL, Mau JL. Antioxidant properties of Agaricusblazei, Agrocybecylindracea, and Boletus edulis. Food Science and Technology. 2007;40(8):1392-1402.
- Chatterjee A, Sharma NJ, Bannerji, Basa SC. Studies on acantheceae-benzoxazolone from Blepharisedulis Pers. Ind J of Chem. 1990;29:132-134.
- Afifi AT. A novel 4'-O-diglycoside of decarboxyrosmarinic acid from Blephariseudalis. Pharma Bio. 2003;41:487-490.
- Chauhan, NS, Dixit VK. Effects of Bryonialaciniosa seeds on sexual behaviour of male rats. Int J of Impotence Res. 2010;22:190-195.
- Reddy J, Vijay GD, Ranganathan TV. In vitro studies on anti asthmatic, analgesic and anti convulsant activities of the medicinal plant Bryonialaciniosalinn. Int J Drug Discovery. 2010;2:1-10.
- Zamble A, Martin-Nizard F, Sahpaz S, Hennebelle T, Staels B, Bordet R. Vaso activity, antioxidant and aphrodisiac properties of Caesalpiniabenthamiana roots. J Ethnopharmacol. 2008;116:112-119.
- Dickson RA, Houghton PJ, Hylands PJ. Antibacterial and antioxidant cassanediterpenoids from Caesalpiniabenthamiana. Phytochemistry. 2007;68(10):1436-1441.
- Rita AD, Peter JH, Peter JH. Antibacterial and antioxidant cassanediterpenoids from Caesalpiniabenthamiana. Phytochem. 2007;68:1436-1441.
- Pande M, Pathak TM. Sexual function improving effect of Chenopodium album (Bathua sag) in normal male mice. Biomed Pharmacol J. 2008;1:325-332.
- Horio TK, Yoshida K, Kikuchi H, Kawabata J, Mizutani J. A Phenolic amide from roots of Chenopodium album. Phytochem. 1993;33:807-808.
- Nahar SD, Sarker A. Chenoalbuside: an antioxidant phenolic glycoside from the seeds of Chenopodium album L (Chenopodiaceae). Braz J of Pharmacol. 2005;15:279-282.
- Thakur M, Chauhan NS, Bhargava S, Dixit VK. A comparative study on aphrodisiac activity of some Ayurvedic herbs Albino rats. Arch Sex Behav. 2009b;38:1009-1015.
- Sharada L, Deore, Somshekhar SK. Isolation and characterization of phytoconstituents from Chlorophytumborivilianum. Pharmacog Res. 2010;2:343-349.
- Acharyaa D, Mitaine-Offera AC, Kaushikb N, Miyamotoc T, Paululatd T, Marie-Aleth LD. Furostane- type steroidal saponins from the roots of ChlorophytumBorivilianum Helvetica 2262. ChimicaActa. 2008;91:211-222.
- Ratnasooriya WD, Fernando TS. Effect of black tea brew of Camellia sinensis on sexual competence of male rats. J Ethnopharmacol. 2008;118:373-377.
- Hsu YW, Tsai CF, Chen WK, Huang CF, Yen CC. A subacute toxicity evaluation of green tea (Camellia sinensis) extract in mice. Food ChemToxicol. 2011;49:2624-30.
- Graham HN. Green tea composition, consumption, and polyphenol chemistry. Preventive Med. 1992;21:334-350.
- Hosseinzadeh H, Ziaee T, Sadeghi A. The effect of saffron, Crocus sativus stigma, extract and its constituents, safranal and crocin on sexual behaviors in normal male rats. Phytomed. 2008;15:491-49.
- Arantilis PA, Tsoupras G, Polissiou M. Determination of saffron (Crocus sativus L) components in crude plant extract using high-performance liquid chromatography-UV-visible photodiode-array detection-mass spectrometry. J Chromatography. 1995;699:107-118.
- Escribano J, Alonso GL, Coca-Prados M, Fernandez JA. Crocin, safranal and picrocrocin from saffron (Crocus sativus L) inhibit the growth of human cancer cells in vitro. Cancer Lett. 1996;100:23-30.
- Lozano P, Delgado D, Gomez D, Rubio M, Iborra JL. A non-destructive method to determine the safranal content of saffron (Crocus sativus L) by supercritical carbon dioxide extraction combined with high-performance liquid chromatography and gas chromatography. J BiochemBiophys Methods. 2000;43:367-378.
- Abe K, Saito H. Effects of saffron extract and its constituent crocin on learning behaviour and long-term potentiation. Phytother Res. 2000;14:149-152.
- Bisset NG, Wichtl M. (2001). Salviaeofficinalis folium Herbal Drugs and Phytopharmaceuticals, A Handbook for Practice on a Scientific Basis With Reference to German Commision E Monographs. 2nd ed. MedpharmStuttgart.pp. 440-443.
- Al-Zubairi AS, Ismail P, PeiPei C, Abdul AB, Ali RS, Wahab ASI. Short-term repeated dose biochemical effects of Catha edulis (Khat) crude extract administration in rats. Int J Trop Med. 2008;3:19-25.
- Al-Meshal IA, Qureshi S, Ageel AM, Tariq M. The toxicity of Catha edulis (khat) in mice. J Subst Abuse. 1991;3:107-15.
- Ali ST, Rakkah NI. Probable neuro sexual mode of action of Casimiroaedulis seed extract verses sildenafil citrate (Viagra tm) on mating behavior in normal male rats. Pak J Pharm Sci. 2008;21:1-6.
- Thakur M, Thompson D, Connellan P, Deseo MA, Morris C, Dixit VK. Improvement of penile erection, sperm count and seminal fructose levels in vivo and nitric oxide release in vitro by ayurvedic herbs. Andrologia. 2011;43:273-7.
- Chauhan NS, Rao CV, Dixit VK. Effect of Curculigoorchioides rhizomes on sexual behaviour of male rats. Fitoterapia. 2007;78:530-534.
- Thakur M, Chauhan NS, Sharma V, Dixit VK, Bhargava S. Effect of Curculigoorchioides on hyperglycemia-induced oligospermia and sexual dysfunction in male rats. Intern J of Impotence Res. 2012;24:31-37.
- Asif M, Kumar A. Acute Toxicity Study and In-vivo Anti-inflammatory Activity of Different Fractions of CurculigoorchioidesGaertn Rhizome in Albino Wistar Rats. Iranian J of Pharmaceut Sci. 2010;6:91-198.
- Garg SN, Misha LN. Corchioside and Orcinol Glycoside from Curculigoorchioides. Phytochem. 1989;28:1771-2.
- Rao KRV, Ali N, Reddy NM. (1978). Occurrence of both Sapogenin and AlkoloidLycorine in Curculigoorchioides. Ind J Pharm Sci.pp.104-105.
- Kubo M, Nakanishi K. A New Phenolic Glucoside, Curculigoside from rhizomes of Curculigoorchioides. Planta Med. 1983;47:52-5.
- Mehata BK, Dubey A. 4-Acetyl-2-methoxy-5-methyltriacetone, a New Aliphatic Long-Chain Methoxyketone from Curculigoorchioides Roots. Indian J Chem. 1983;22:282-3.
- Mehata BK, Gawarikar R. Characterization of Novel Triterpenoid from Curculigoorchioides. Indian J Chem. 1991;30:986-9.
- Romero ML, Escobar LI, Lozoya X, Enríquez RG. High-performance liquid chromatographic study of casimiroaedulis: I Determination of imidazole derivatives and rutin in aqueous and organic extracts. J of Chromatography. 1983;281:245-251.
- Kizu H, Kaneko E, Tomimori T. Studies on Nepalese Crude Drugs XXVI1 Chemical Constituents of PanchAunle, the roots of Dactylorhizahatagirea D DON. Chem Pharm Bull. 1999;47:1618-1625.
- Venkatesh P, Hariprasath K, Soumya V, Francis MP, Sankar S. Isolation and aphrodisiac screening of the fruits of Duriozibenthinus. Linn Asian J Bio Sci. 2010;3:1-9.
- Sunanta P, Suchada S, Achara T. The determination of toxic effects at a high oral dose of polysaccharide gel extracts from fruit-hulls of durian (Duriozibethinus L) in mice and rats. Songklanakarin J of Sci and Tech. 2001;23:55-62.
- Drewes SE, Horn MM, Munro QQ, Dhlamini JT, Meyer JJ, Rakuambo NC. Pyrano-isoflavones with erectile-dysfunction activity from Eriosemakraussianum. Phytochem. 2002;59:739-47.
- Ang HH, Cheang HS. Effects of Eurycomalongifolia Jack on laevatorani muscle in both uncastrated and testosterone stimulated castrated intact male rats. Arch Pharm Res. 2001;24:437-440.
- Zanoli P, Zavatti M, Montanari C, M Baraldi. Influence of Eurycomalongifolia on the copulatory activity of sexually sluggish and impotent male rats. J Ethnopharmacol. 2009;126:308-313.
- Ang HH, Ngai TH, Tan TH. Effects of Eurycomalongifolia Jack on sexual qualities in middle aged male rats. Phytomed. 2003;10:590-593.
- Ang HH, Sim MK. Eurycomalongifolia Jack and orientation activities in sexually experienced male rats. Biol Pharm Bull. 1998;21:153-155.
- Ang HH, Chan KL, Gan EK, Yuen KH. Enhancement of sexual motivation in sexually naive male mice by Eurycomalongifolia. Int J Pharmacog. 1997;35:144-146.
- Kardono LBS, Angerhofer CK, Tsauri S, Padmawinata K, Pezzuto LM, Kinghorn ADJ. Cytotoxic and antimalarial constituents of the roots of Eurycomalongifolia. J Nat Prod. 1991;54:1360-7.
- Morita H, Kishi E, Takeya K, Itokawa H, Iitaka Y. Highly oxygenated quassinoids from Eurycomalongifolia. Phytochem. 1993;33:691-6.
- Itokawa, H, Qin XR, Morita H, Takeya KJ. C18 and C19 quassinoids from Eurycomalongifolia. J Nat Prod. 1993;56:1766-71.
- Ang HH, Hitotsuyanagi Y, Fukaya H, Takeya K. Quassinoids from Eurycomalongifolia. Phytochem. 2002:59:833-7.
- Bedir E, Abou-Gazar H, Ngwendson JN, Khan IA. Eurycomaoside: a new quassinoid-type glycoside from the roots of Eurycomalongifolia. Chem Pharm Bull. 2003;51:1301-3.
- Kuo, PC, AGDamu, KH Lee, TS Wu. Cytotoxic and antimalarial constituents from the roots of Eurycomalongifolia. Bioorganic and Med Chem. 2004;12:537-544.
- El-Taher TS, Matalka Z, Taha HA, Badwan AA. Ferula harmonis `zallouh' and enhancing erectile function in rats: Efficacy and toxicity study. Int J Impot Res. 2001;13:247-251.
- Ahmed AA. Sesquiterpenecoumarins and sesquiterpene from Ferula sinaica. Phytochem. 1999;50:109-112.
- Chaturapanich G, Chaiyakul S, Verawatnapakul V, Pholpramool C. Effects of Kaempferiaparviflora extracts on reproductive parameters and spermatic blood flow in male rats. Reproduction. 2008;136:515-522.
- Sudwan P, Saenphet K, Saenphet S, Suwansirikul S. Effects of Kaempferiaparviflora Wall Ex Baker on sexual activity in male rats and its toxicity. Southeast Asian J Trop Med Public Health. 2006;37:210-5.
- Temkitthawon P, Hinds TR, Beavo JA, Viyoch J, Suwanborirux K, Pongamornkul W, et al. Kaempferiaparviflora, a plant used in traditional medicine to enhance sexual performance contains large amounts of low affinity PDE5 inhibitors. J Ethnopharmacol. 2011;137:1437-41.
- Luo Q, Li Z, Huang X, Yan J, Zhang S, Cai YZ. Lyciumbarbarum polysaccharides: Protective effects against heat-induced damage of rat testes and H2O2-induced DNA damage in mouse testicular cells and beneficial effect on sexual behavior and reproductive function of hemicastrated rats. Life Sci. 2006;79:613-621.
- Xie, C, Xu LZ, Li XM, Li KM, Zhao BH, Yang SL. Studies on chemical constituents in fruit of Lyciumbarbarum L. ZhongguoZhong Yao ZaZhi. 2001;26:23-4.
- Carro-Juárez M, Cervantes E, Cervantes-Méndez M, Rodríguez-Manzo G. Aphrodisiac properties of Montanoatomentosa aqueous crude extract in male rats. PharmacolBiochemBehav. 2004;78:129-34.
- Fred C, Seaman A, Malcolm AJ, Nikolaus HF. Tomexanthin, an oxepanediterpene from Montanoatomentosa. Phytochem. 1984;23:464-465.
- Robles -Zepeda, Molina-Torres J, Lozoya-Gloria E, Lopez MG. Volatile organic compounds of leaves and flowers of Montanoatomentosa. Flavour and Fragrance J. 2006;21:225-227.
- Suresh S, Prithiviraj E, Prakash S. Dose- and time-dependent effects of ethanolic extract of Mucunapruriens Linn seed on sexual behaviour of normal male rats. J Ethnopharmacol. 2009;122:497-501.
- Kumar A, Rajput G, Kumar VD, Srivastav G. Phytocontent Screening of Mucuna Seeds and Exploit in Opposition to Pathogenic Microbes. J Biol Environ Sci. 2009;3(9):71-76.
- Yakubu MT, Awotunde OS, Ajiboye OT, Oladiji AT, Akanji MA. Pro-sexual effects of aqueous extracts of Massulariaacuminata root in male Wistar rats. Andrologia. 2008;43:334-340.
- Yakubu MT, Akanji MA. Effect of Aqueous Extract of Massulariaacuminata Stem on Sexual Behaviour of Male Wistar Rats. Evid Based Complement Alternat Med. 2011;2011:738103.
- Tajuddin A, Ahmad S, Latif A, Qasmi IA, Amin KMY. An experimental study of sexual function improving effect of MyristicafragransHoutt (Nutmeg). BMC Complement Altern Med. 2005;5:16-21.
- Shukla J, Tripathi SP, MK Chaubey. Toxicity of Myristicafragrans and Illiciumverum essential oils against flour-beetle TriboliumcastaneumHerbst (Coleoptera: Tenebrionidae). Electronic J Environ Agric Food Chem. 2008;7:3059-3064.
- Isogai, A, Suzuki A, S Tamura. Structure of dimericphenoxy-propanoids from Myristicafragrans. Agar Biol Chem. 1973;37:193-194.
- Zamble A, Martin-Nizard F, Sahpaz S, Reynaert ML, Staels B, Bordet R, et al. Effects of Microdesmiskeayanaalkaloids on vascular parameters of erectile dysfunction. Phytother Res. 2009;23:892-5.
- Zamble A, Sahpaz S, Hennebelle T, Carato P, Bailleul F. N1,N5,N10-Tris(4- hydroxycinnamoyl) spermidines from MicrodesmiskeayanaRoots. Chem and Biodiver. 2006;3:982-989.
- Zamble A, Hennebelle T, Sahpaz S, Bailleul F. Two new quinoline and tris(4-hydroxycinnamoyl) spermine derivatives from Microdesmiskeayana roots. Chempharmaceutic bull. 2007;55:643-645.
- Sekar S, Elumalai P, Seppan P. Dose- and time-dependent effects of ethanolic extract of Mucunapruriens Linn. seed on sexual behaviour of normal male rats. Journal of Ethnopharmacology. 2009;122(3):497-501.
- Suresh S, Prakash S. Effect of Mucunapruriens (Linn.) on sexual behavior and sperm parameters in streptozotocin-induced diabetic male rat. J Sex Med. 2012;9:3066-3078.
- Uchegbu RI, JohnBullOnyekachiEcheme. (2013). Isolation and Characterization of Estra–2ll–en –17–ol, 3yl benzoate from Mucunapruriens (Utilis). Uchegbu J of Natural sciencsResearch.Vol. 3.
- Orafidiya LO, Agbani EO, Iwalewa EO, Adelusola KA, Oyedapo OO. Studies on the acute and sub-chronic toxicity of the essential oil of Ocimumgratissimum L leaf. Phytomed. 2004;11:71-6.
- Matasyoh LG, Josphat CM, Francis NW, Miriam GK, Anne WTM, Titus KM. Chemical composition and antimicrobial activity of the essential oil of Ocimumgratissimum L growing in Eastern Kenya. Afr J Biotech. 2007;6:760-5.
- Janine AL, Xisto SP, Orionalda FLF, José RP, Pedro HF, Lúcia KHS, et al. Antifungal activity from Ocimumgratissimum L towards Cryptococcus neoformans. MemInstOswaldo Cruz. 2005;100:55-8.
- Dubey NK, Kishore N, Varma J, Lee SY. Cytotoxicity of the essential oils of Cymbopogoncitratus and Ocimumgratissimum. Indian J Pharm Sci. 1997;59:263-4.
- Jirovetz, L, Buchbauer G, Ngassoum MB, Ngamo LT, Adjoudji O. Combined investigation of the chemical composition of essential oils of Ocimumgratissimum and Xylopiaaethiopica from Cameroon and their insecticidal activities against stored maize pest Sitophiluszeamais. Ernähr. 2005;29:55-60.
- Njoku CJ, Zeng L, Asuzu IU, Oberlies NH, Mclaughlin JL. Oleanolic acid, a bioactive component of the leaves of Ocimumgratissimum (lamiaceae). Int J Pharmacognosy. 1997;35:134-7.
- Chen X. Cardiovascular protection by ginsenosides and their nitric oxide releasing action. ClinExpPharmacol Physiol. 1996;23(8):72-732.
- Gillis CN. Panax ginseng pharmacology: A nitric oxide link? BiochemPharmacol. 1997;54:1-8.
- Balamurugan G, Muralidharan P, Palapala S. Aphrodiasic activity and curative effect of Pedalium murex (L) against ethanol induced infertility in male rats. Turk J Biol. 2010;34:153-163.
- Subramanian SS, Nair AGR. Flavonoids of the leaves of Pedalium murex. Phytochem. 1972;11:464.
- Yogendra N, Raghunath S, Thakur S. Hepta triacontan-4-1, tetratriacontanyloctacosanoate and other constituents from P murex. Phytochem. 1983;22:973-974.
- Subhan F, Sultan S, Alam W, Tahir F, Dil AS. Aphrodisiac potential of Peganumharmala seeds. HamdardMedicus. 1998;4:69-72.
- Sharaf M, El-Ansari EA, Matlin SA, Saleh NAM. Four flavonoid glycosides from Peganumharmala. Phytochem. 1997;44:533-536.
- Abdel-Aziz, HG, Abdel- Kader SM, El-Sayed MM, EL-Malt EA, Shaker ES. Novel beta-carboline alkaloid from Peganumharmala as antibacterial agent. Tenth Radiation Physics and Protection Conference. 2010;4(1):27-30.
- Dhawan K, Kumar S, Sharma A. Aphrodisiac activity of methanol extract of leaves of Passifloraincarnata Linn in mice. Phytother Res. 2003;17:401-403.
- Anita SP. Exploring Passifloraincarnata (L): A medicinal plants secondary metabolites as antibacterial agent. J of Med Plants Res. 2006;4:1496-1501.
- Li QM, Van den HH, Delorenzo O, Corthout J, Pieters LA, Vlietinck AJ, et al. Department of Pharmaceutical Sciences, University of Antwerp (UIA), Wilrijk-Antwerp, Belgium Mass spectral characterization of C-glycosidic flavonoids isolated from a medicinal plant (Passifloraincarnata). J of Chromatography. 1991;562:435-46.
- Abdullah A, Qarawi A. Stimulatory effect of the aqueous extract of Rutachalepensis on the sex organs and hormones of male rats. J Appl Res. 2005;5:206.
- Gonzalez-Trujano ME, Carrera D, Ventura-Martinez R, Cedillo-Portugal E, Navarrete A. Neuropharmacological profile of an ethanol extract of Rutachalepensis L in mice. J of Ethnopharmacol. 2006;106:129-135.
- Al-Said MS, Tariq M, Al-Yahya MA, Rafatullah S, Ginnawi OT, Ageel AM. Studies on Rutachalepensis, an ancient medicinal herb still used in traditional medicine. J of Ethnopharmacol. 1990;28:305-12.
- Meyer JJM, Rakuambo NC, Hussein AA. Novel xanthones from Securidacalongepedunculata with activity against erectile dysfunction. J Ethnopharmacol. 2008;119:599-603.
- Etuk EU, Adebiyi RA, Elsa AT, Agaie BM. Acute and Subchronic (28-day) Oral Toxicity Studies of the Aqueous Root Extract of SecuridacalongepedunculataFresen (Polygalaceae) in Mice. Int J Pharmacol. 2006;2:421-42.
- Sharma V, Boonen J, Chauhan NS, Thakur M, De Spiegeleer B, Dixit VK. Spilanthesacmellaethanolic flower extract: LC-MS alkylamide profiling and its effects on sexual behavior in male rats. Phytomed. 2011;18:1161-9.
- Mishra RK, Singh SK. Safety assessment of Syzygiumaromaticum flower bud (clove) extract with respect to testicular function in mice. Food ChemToxicol. 2008;46:3333-3338.
- Nassar IM, Gaara AH, El-Ghorab AH, Abdel-Razik HF, Shen H, Huq E, et al. Chemical Constituents of Clove (Syzygiumaromaticum, famMyrtaceae) and their antioxidant activity. Rev LatinoamerQuím. 2007;35:41-50.
- Kumar S, Madaan R, Sharma A. Evaluation of Aphrodisiac Activity of Turneraaphrodisiaca. Intern J of Pharmacog and Phytochem Res. 2009;1:1-4.
- Suresh K, Subramoniam A, Pushpangadan P. Aphrodisiac Activity Of Vanda Tessellata (Roxb) Hook Ex Don Extract In Male Mice. Ind J Pharmacol. 2000;32:300-304.
- Spencer KC, Seigler DS. Cyanogenic Glycosides of Carica papaya and its Phylogenetic Position with Respect to the Violales and Capparales. American J of Bot. 1981;71:1444-1447.
- Dominguez XA, Hinojosa M. Mexican medicinal plants XXVIII Isolation of 5-hydroxy- 7-3- trimethoxy flavones from T diffusa. Planta Med. 1976;30:68-71.
- Auterhoff H, Hackle HP. Components of Damiana drug. Arch Pharm. 1968;301:537-544.
- Dixit SN, Khosa RL. Chemical investigation on Tinosporacordifolia. Ind J of App Chem. 1971;34:46-47.
- Hanuman JB, Bhatt RK, Sabata BK. A clerodanefurano-diterpene from Tinosporacordifolia. J Nat Prod. 1988;51(2):197.
- Maurya R, Handa SS. Tinocordifolin, a sesquiterpene from Tinosporacordifolia. Phytochem. 1998;49:11343-6.
- Estrada-Reyes R, Ortiz-Lopez P, Gutierrez-Ortíz J, Martínez-Mota L. Turneradiffusa Wild (Turneraceae) recovers sexual behavior in sexually exhausted males. J Ethnopharmacol. 2009;123:423-9.
- Andreia G, Bezerra R, Fulvio MRT, Carlini EA. Effects of a hydroalcoholic extract of T fiffusa in tests for adaptogenic activity. Bra J Pharmacol. 2011;21:121-127.
- Zhao J, Pawar RS, Ali Z, Khan IA. Phytochemical investigation of Turneradiffusa. J Nat Prod. 2007;26:289-92.
- Padashetty SA, Mishra SH. Aphrodisiac studies of Tricholepisglaberrima with supportive action from antioxidant enzymes. Pharm Biol. 2007;45:580-586.
- Gauthaman K, Adaikan PG, Prasad RN. Aphrodisiac properties of TribulusTerrestris extract (Protodioscin) in normal and castrated rats. Life Sci. 2002;71:1385-1396.
- Neychev VK, Mitev VI. The aphrodisiac herb Tribulusterrestris does not influence the androgen production in young men. J Ethnopharmacol. 2005;101:319-323.
- Tyagi RM, Aswar UM, Mohan V, Bodhankar SL, Zambare GN, Thakurdesai PA. Study of furostenol glycoside fraction of Tribulusterresteris on male sexual function in rats. Pharm Biol. 2008;46:191-198.
- Gauthaman K, Ganesan AP, Prasad RN. Sexual effects of puncturevine (Tribulusterrestris) extract (protodioscin): an evaluation using a rat model. J Altern Complement Med. 2003;9:257-265.
- Tian-Shung W, Li-Shian S, Shang-Chu K. Alkaloids and other constituents from Tribulusterrestris. Photochem. 1999;50:1411-1415.
- Kostovaa I, Dincheva D, Rentschb GP, Dimitrovb V, Ivanovaa A. Two New Sulfated FurostanolSaponins from Tribulusterrestris. Z Naturforsch. 2002;57:33D-38.
- Subramoniam A, Madhavachandran V, Rajasekharan S, Pushpangadan P. Aphrodisiac property of Trichopuszeylanicus extract in male mice. J Ethnopharmacol. 1997;57:21-27.
- Chacko S, Sethuraman MG, Gorge V, Pushpangadan P. Phytochemical constituent of Trichopuszeylanicus. J Med and Aromatic Plant Sci. 2002;24:703-706.
- Abbas AM, Kamla K, Mohammad KA, Singh R, Satya NS, Singh V, et al. Withaniasomnifera Improves Semen Quality in Stress-Related Male Fertility. Evidence-Based Complementary and Alternative Medicine. 2011;2011:9.
- Matsuda H, Murakami T, Kishi A, Yoshikawa M. Structures of withanosides I, II, III, IV, V, VI, and VII, new withanolide glycosides, from the roots of Indian Withaniasomnifera DUNAL. and inhibitory activity for tachyphylaxis to clonidine in isolated guinea-pig ileum. Bioorganic & Medicinal Chemistry. 2001:9(6);1499-1507.









