e-ISSN: 2322-0139 p-ISSN: 2322-0120
e-ISSN: 2322-0139 p-ISSN: 2322-0120
Mishra Pankaj*, Saxena Vikas and Saxena Abhishek
Department of Pharmaceutical Chemistry, Rakshpal Bahadur College of Pharmacy, Bareilly – 243001, Uttar Pradesh, India
Received date: 23/03/2014 Revised date: 02/05/2014 Accepted date: 09/05/2014
Visit for more related articles at Research & Reviews: Journal of Pharmacology and Toxicological Studies
In the substituted Cinnoline Imidazole series, the compounds which are halogen mainly Chloro Substituted were showed potent antibacterial, anti-inflammatory and anti-fungal activity than other compounds. However Methyl substituted compound also showed more potent antimicrobial activity and antiinflammatory activity.
Cinnoline, Imidazole, Anti Inflamatory, Anti-bacterial
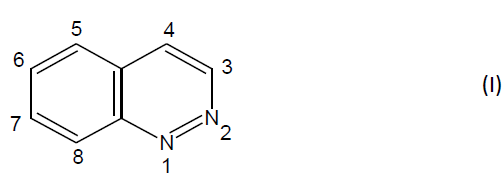
Cinnoline is a pale yellow solid, m.p. 24-25°C and was first discovered by Von Richter in 1883. He also prepared a cinnoline derivative from 2-aminophenylpropionic acid via intramolecular cyclization of the diazonium salt. The review of literature showed that cinnoline derivatives were found to elicit many pharmacological actions like anti-hypertensive, antithrombotic, antihistamine, antileukemic, CNS activity, anti-tumor, antibacterial and antisecretory activity. They are reactive by virtue of the presence of a benzene ring and the electrophillic attack takes place in this ring. Cinnolines are the six-membered heterocyclic compounds having two hetero atoms in the ring. They are also called as 1, 2- benzodiazine or benzopyridazine or 1, 2- diazanaphthalene or phenodiazine [1-10]. (I)
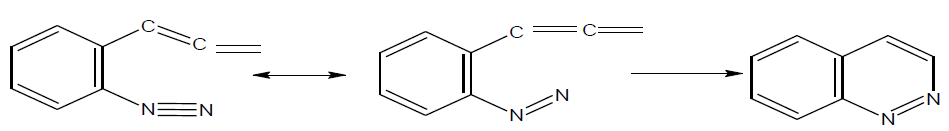
The main approach for the synthesis of cinnoline is electrophilic attack by diazonium cation on carbon – carbon center of unsaturation as given below.
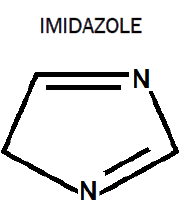
Imidazole is an organic compound with the formula C3H4N2. This aromatic heterocyclic is a diazole and is classified as an alkaloid. Imidazole refers to the parent compound whereas imidazoles are a class of heterocycle with similar ring structure but varying substituents. This ring system is present in important biological building blocks such as histidine, and the related hormone histamine. Imidazole can serve as a base and as a weak acid. Many drugs contain an imidazole ring, such as antifungal drugs and nitroimidazole.
During the past decade, imidazole derivatives have occupied a unique place in the field of medicinal chemistry. They have wide range of biological activities. They are well known analgesics, anti-inflammatory, antiparasitic, anthelmintic, platelet aggregation inhibitors and antiepileptic agents. Imidazole can be found in many other drugs such as dacarbazine, metronidazole, cimetidine, flumazenil, thyroliberin, methimazole, pilocarpine and etomidate which are used as antineoplastic antibiotic, antiulcerative, benzodiazepine antagonist, prohormone, antihyperthyroid, muscarinic receptor [11-23].
Biological Evaluation
Antibacterial activity studies
The newly synthesized different series of substituted cinnoline derivatives in the present investigations were tested for their antibacterial activities.
General Procedure
Disk diffusion method
Modified Kirby-Bauer method was used for the evaluation of microbial sensitivity of the synthesized compounds. Circular paper disks were impregnated with the specific amount of test compounds and were placed on suitable agar medium (Muller Hinton agar), which was inoculated with the test organism. After incubation the petri dishes were observed for growth of inhibition zone around the disk. A “halo” or Zone of inhibition forms, where concentration of the diffused molecule is sufficient to inhibit microbial growth. The diameter of zone of inhibition is directly proportional to antimicrobial activity of the compound. The diameter of zone of inhibition was compared with that of standard antibiotics.
MIC (Minimum Inhibitory Concentration)
MIC of the synthesized compounds was determined by tube dilution techniques. Serial dilution of the substance under examination was placed into culture tubes containing suitable medium and inoculated with the test organism. After incubation, the minimum concentration of test compound that inhibited the growth of the organism was observed.
Anti-inflammatory Activity
The anti-inflammatory activity was assessed by rat paw edema method wherein the procedure of plethysmographic measurement of edema produced by planter injection of 1% w/v formalin in the hind paw of the rat was followed. The method described by Wilhelm and Domenoz as modified by Sisodia and Rao was used for measuring the paw volume. Suspension of phenylbutazone containing 40 mg/ml of drug was prepared in 2% gum acacia and used as standard drug. Suspensions of test compounds at a concentration of 40 mg/ml were also prepared in 2% gum acacia. The dose concentration of both standard drug and the test compounds was 100 mg/kg body weight. 1% w/v of formalin solution prepared and 0.1 ml of it in each case was injected in the planter region of left hind paw of albino rats.
Albino rats of either sex weighing 150-200 grams were used and divided into groups of six albino rats in each group. First group served as control, second group was used for standard drug phenylbutazone and the remaining groups served for compounds under investigation. An identification mark was made on both the hind paws just beyond tibiotorsal junction so that every time the paw was dipped in mercury column upto a fixed mark to ensure constant paw volume. Immediately after 30 minutes of drug administration, 0.1 ml of 1% w/v formalin was injected in the planter region of left paw of the rats. The right paw was used as reference for non inflammated paw for comparision. The paw volume of all the test animals was measured after 2nd and 4th hours of drug administration. The percentage of increase in edema over the initial reading was also calculated. The increase in edema of animals treated with standard test compounds were compared with the increase in the edema of untreated control animal with the corresponding intervals of 2nd and 4th hours. Thus the percentage inhibition of edema at known intervals in treated animals was calculated as given below.
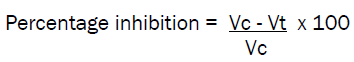
Vc = volume of paw edema in control animals Vt = volume of paw edema in treated animals
Data analysis
The data were subjected to analysis of variance (ANOVA) as per statistical methods using SPSS (1996) software package.
Experimental Work
The synthesis of substituted cinnoline Imidazole derivatives by the described above method remitted in products with good yield.
Biological Evaluation (SCHEME - 15a-j) Antibacterial activity studies Disk Diffusion Method
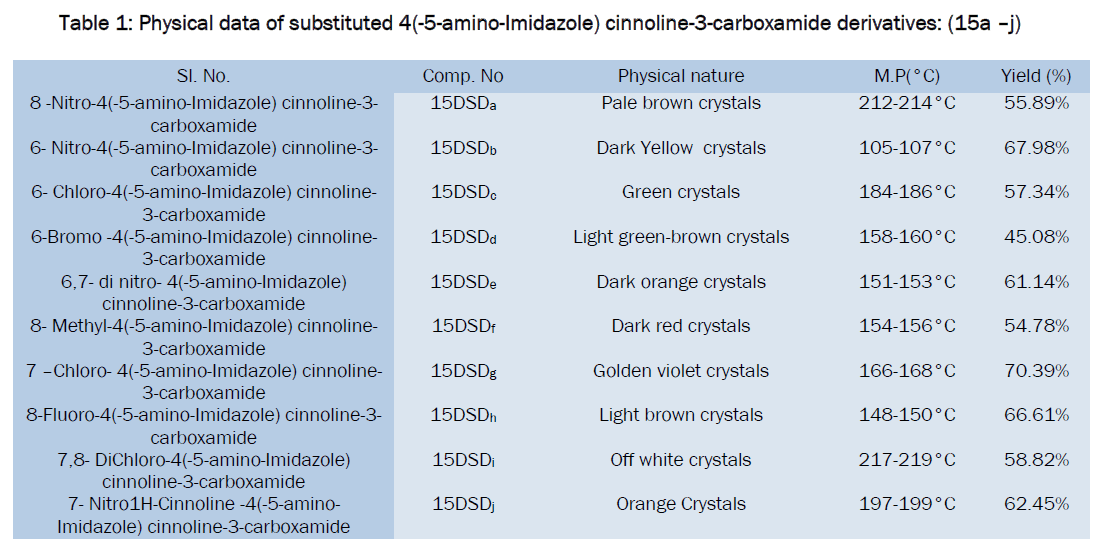
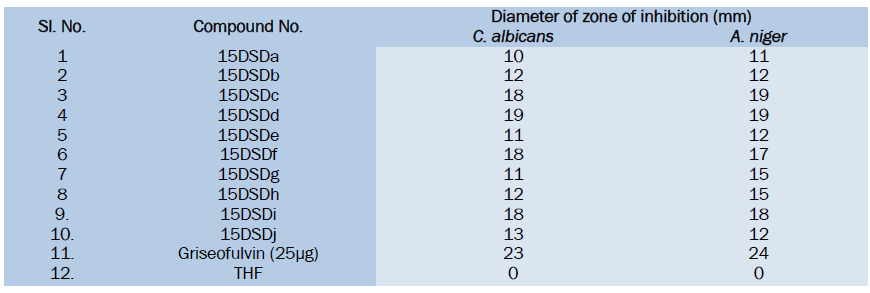
Table Data for antibacterial activities of synthesized compounds
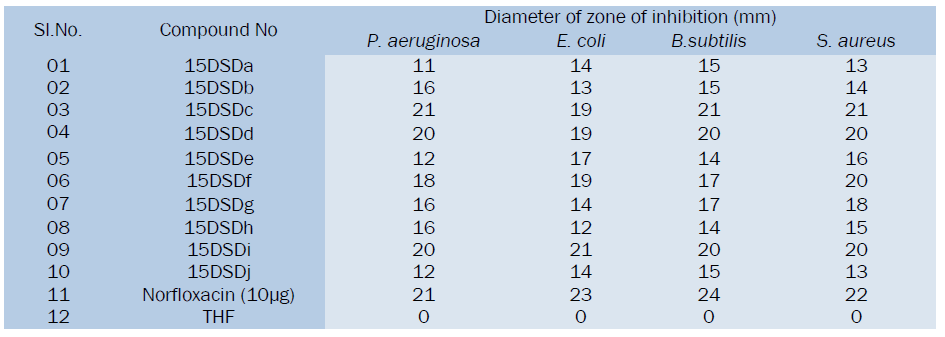
All the synthesized substituted cinnoline Imidazole derivatives were tested at 50μg level and shown moderate to good antibacterial activity, among the tested compounds 15DSDc, 15DSDd, 15DSDf and 15DSDi showed significant activity while other compounds showed moderate activity in comparison with the standard drug norfloxacin.
MIC (Minimum Inhibitory Concentration)
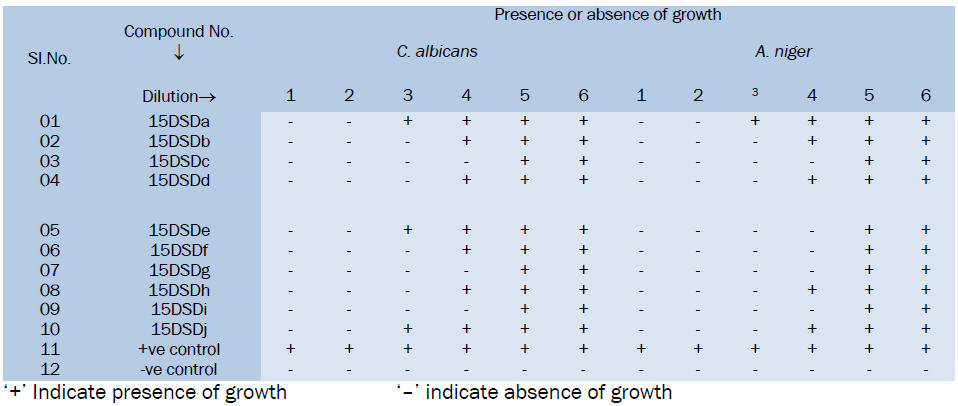
Data of MIC for anti-bacterial activity
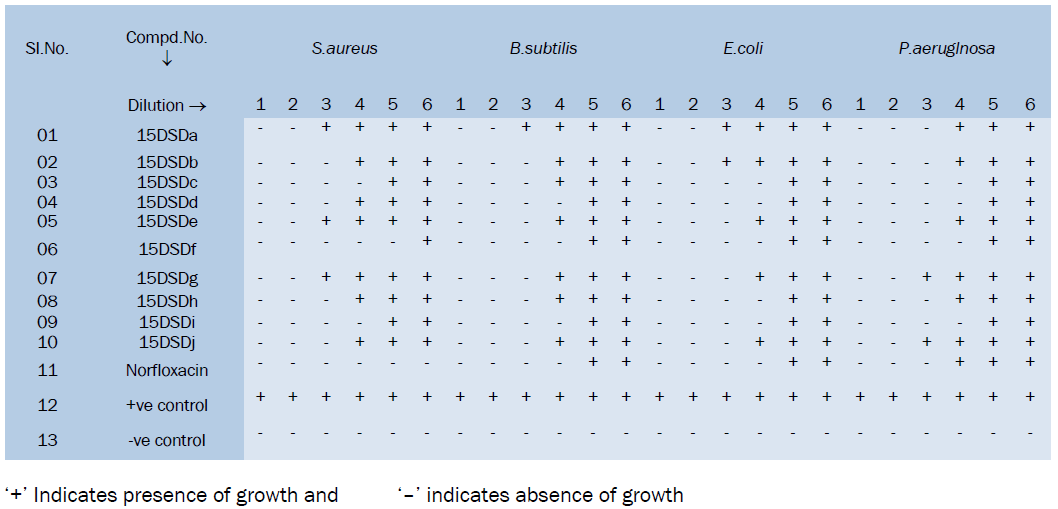
‘+’ Indicates presence of growth and ‘–’ indicates absence of growth
The concentration of derivatives in different dilution is given below:
Derivatives like 15DSDc, 15DSDd, 15DSDf, 15DSDi showed higher activity than all. These compounds can be subjected to further studies for toxicity.
Anti-Fungal Activity Studies Disk diffusion method
Table: Data for antifungal activity of synthesized compounds
All the Substituted Cinnoline Imidazole derivatives were tested at 50μg level. From the anti-fungal activity studies it is evident that the synthesized compounds showed moderate to good anti-fungal activity. Among the tested compounds 15DSDc, 15DSDd, 15DSDf, 15DSDi have shown good activity against C. albicans and A.niger. While other compounds shown weak anti-fungal activity in comparison with the standard drug griseofulvin.
MIC (Minimum Inhibitory Concentration)
Table: Data of MIC for antifungal activity.
‘+’ Indicate presence of growth ‘–’ indicate absence of growth
All the Synthesized compounds have shown anti-fungal activity to a certain extent. Among the tested compounds 15DSDc, 15DSDf and 15DSDi have shown good activity against C. albicans and A.niger. while other compounds show moderate activity.
Result of Anti-inflammatory Activity
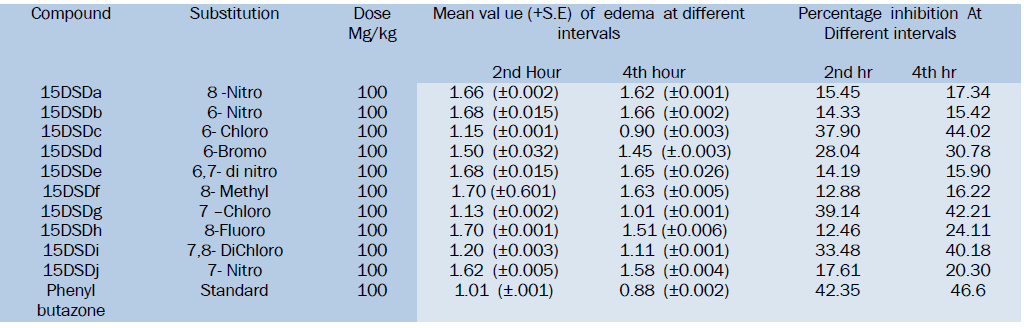
All the Synthesized compounds have shown anti-inflammatory activity to a certain extent as compared to standard drug Phenylbutazone. Among the tested compounds 15DSDc, 15DSDf and 15DSDi have shown good activity by formalin induced rat paw edema method.
Results Characterization
The characterization requires the identification of molecular frame work, the nature of functional groups that are present and their location within the skeletal structure and finally the establishment of any stereo chemical relationships, which might exist.
The problem of characterization of organic compounds has been revolutionized by the progressive adoption of the wide range of spectroscopic techniques, which are now available. These have been applied extensively in the preparative section to confirm the structure of the expected products. The same were applied in present work to confirm the structure of newly synthesized compounds.
In the present work the representative products were characterized by their infrared (IR) spectra, proton magnetic resonance (PMR) spectra and mass spectra. Some intermediates were characterized by measuring their melting point and comparing with literature value, wherever possible.
The IR spectra were recorded by NICOLETT-IMPACT-400FT-IR SPECTRO PHOTOMETER using a thin film supported on KBr pellets.
The PMR spectra were recorded on JEOL-JMS D-300 (300 MHz) NMR spectro meter. All spectra were obtained in Deuturated Methanol and chemical shift values are reported as values in ppm relative to TMS (? = 0) as internal standard.
Mass spectra were recorded on JEOL SX102 MS System operating at 70 ev.
The IR, NMR and MASS spectra of one Compound from each Series is given in figure 15.1 to 15.3 for the representative compounds.
Sample 15DSDi : C.No. 15DSDi _ 7,8-Di-chloro-4(-5-amino- Imidazole) cinnoline-3-carboxamide
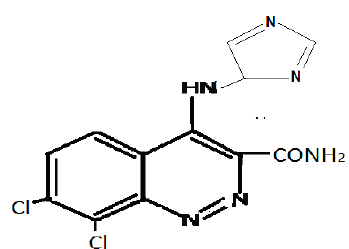
IR (KBr) in cm-1
Peak at 3466.1 cm-1 corresponds to NH stretching Peak at 3341.5 cm-1 corresponds to asymmetric NH2 group. Peak at 3236.2 cm-1 corresponds to CH stretching. Peak at 1671.9 cm-1 corresponds to C = O stretching. Peak at 1500.6 cm-1 corresponds to aromatic C = C stretching. Peak at 1671.9 cm-1 corresponds to C = N stretching. Peak at 1208 - 1671 cm-1 corresponds to imidazole
H1-NMR δ in ppm
δ 8.10 – 8.25 (2H, d, of cinnolines) δ 7.51 – 7.67 (3H, d, Imidazole) δ 14.11 (1H, s, of NH) δ 10.35 (2H, s, of CONH2)
Mass in m/z
Molecular ion peak at m/z = 323 mHz is because of molecular formula C12H8C12N6O. Base peak is at m/z = 154 mHz. Fragment ion peak is observed at m/z = 256 because of C9H5C12N4O, m/z = 241 because of C9H4C12N3O, m/z =82 because of C3H4N3.