e-ISSN: 2319-9849
e-ISSN: 2319-9849
Department of Chemistry, College of Science, Sultan Qaboos University, Box 36, Al-Khodh 123, Sultanate of Oman.
Received: 25 November 2013; Revised: 10 December 2013; Accepted: 18 December 2013
Visit for more related articles at Research & Reviews: Journal of Chemistry
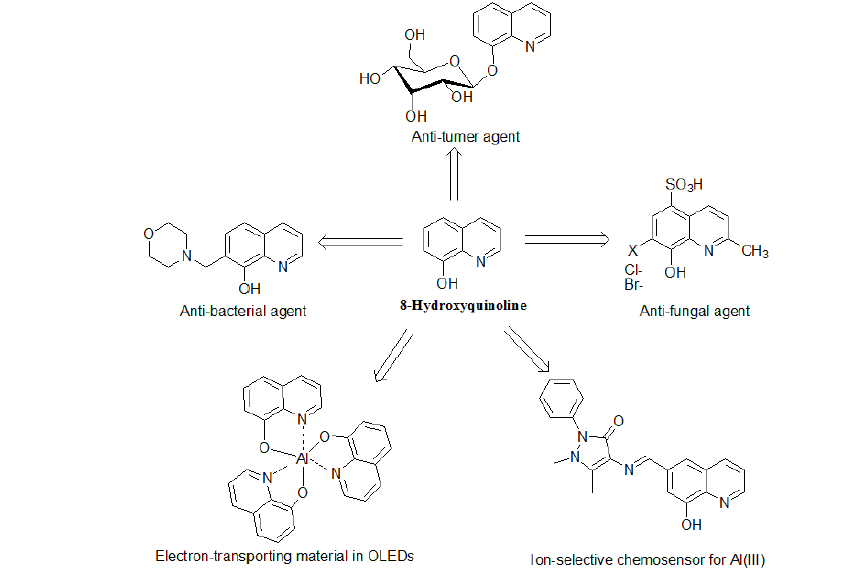
Studies of structural, chemical and synthetic aspects of 8-hydroxyquinoline (8-HQ) and its derivatives are reviewed. A special emphasis is given to applications of recently developed 8-HQ derivatives in various fields such as OLEDs, chemosensors, medicinal drugs, and insecticidal agents.
8-Hydroxyquinoline; OLEDs; Suzuki reaction;Fluorescence sensors; Aluminum ion.
8-Hydroxquinoline(8-HQ), one of the most popular and versatile organic compound, is an organic crystalline material that made up of two rings: a phenol ring fused with pyridine ring. 8-Hydroxyquinoline and its derivatives have found a great variety of applications ranging from pharmacological and pharmaceutical agents to electron carriers in organic light-emitting diodes (OLEDs) and fluorescent chemosensors for metal ions. In medicinal field, 8-hydroxyquinoline derivatives can be used as insecticides, antibacterial, fungicidal, neuroprotective, and anti-HIV agents. In addition, due to their chelating ability toward a great number of metal cations, derivatives of 8-HQ have found many applications in fluorescent sensing of biological and environmentally important metal ions such as Al3+ and Zn2+.8-hydroxyquinoline ligand has been applied for analytical purposes and separation techniques, it is an excellent reagent for gravimetric analysis and it can be used for extraction of metal ions. As it is well known that 8-hydroxyquinoline is weakly fluorescent because of the excited state intermolecular proton transfer of the proton of the OH-function to the nitrogen of the pyridine moiety; chelating metals ions react with 8-hydroxyquinoline to increase the fluorescence emission of this ligand greatly. This increase in the emission appears to be largely a result of the increased rigidity in the molecule.This article will focus on the structural, physical properties and chemical properties aspects of 8-HQ derivatives and their metal complexes. Furthermore, particular attention is paid to the applications of 8-HQ derivatives in various fields.
8-hydroxyquinoline (8-HQ)1 is a bicyclic compound derived from quinoline2(1-azanaphthalene) and consist of two ringssystem: carbocylic ring and pyridine ring with hydroxyl group substituted at position-8 (Figure-1).8-HQ has typical phenolic properties, e.g. it give violet colour with ferric chloride, couple with diazoniumcations, and participate in Reimer-Tiemann and Bucherer reactions; its acetate ester usually undergo the Fries rearrangement with aluminium chloride to give acetyl derivative [1].
Like other hydroxyquinolines, 8-HQ form an equilibrium mixture of the hydroxyl-form 1 and the N-protonated zwitterionic form 3 as shown in (Figure-2) [2].
As a result of the proximity of the hydroxyl group to the heterocyclic nitrogen, 8-HQ forms insoluble chelate complexes with a great variety of metal ions, including Cu2+, Bi2+, Mn2+, Mg2+, Fe3+, Al3+, Zn2+ and Ni3+ [3]. The hydrogen of the hydroxyl group in 8-HQ is displaced and the metal is linked to both the oxygen and nitrogen. Fourcovalent metal complexes therefore require two molecules of 8-HQ for each atom of metal (structure-4, Figure-3) and six-covalent metal complexes require three moleculesof 8-HQ (structure-5, Figure-3).
Synthesis Of 8-Hydroxyquinoline And DerivativesThree main synthetic methods are available to prepare 8-HQ and its derivatives:
Via Constructing the Heterocyclic Ring
8-HQ and its derivatives areusually synthesized by Skraupor Friedlander methods [4]. In the first reaction, a readily available substituted aromatic aminesreact with α,β-unsaturated aldehyde; whilein the second method derivative of 8-HQ are formed by the condensation of substituted o-aminobenzaldehyde or o-aminoacetophenone with suitable aldehyde or ketone (Scheme-1).
Introducing –OH Group to the Quinoline Skeleton
8-HQ and derivatives can be synthesized by diazotization of 8-aminoquinoline 6 or from 8-sulphonic acid 7by alkali fusion (Scheme-2) [5].
By Introducing Substitutions to 8-HQ Skeleton
The most used method in this category is Suzuki cross-coupling reactionin which a new substitution can be introduced at position 5 only or at positions 5 and 7 of the 8-HQ moiety (Scheme-3).The synthesis of 5-aryl-8-HQ 8 commences from 5-bromo-8-HQ 9 and requires protection of the 8-OH group prior to Suzuki coupling reaction. The usual protection group used is benzyl group which is easily removed by catalytic hydrogen transfer from cyclohexa- 1,4-diene (Scheme-3) [6].On the other hand, preparation of 5,7-disubstituted 8-HQ starts with 5,7-dibromo-8-HQ [7]. In addition, 4-Aryl-8-HQ 10can also be prepared from 4-chloro-8-tosyloxyquinoline 11 using the above method with different de-protection method [8].
8-Hq Derivatives As Bioactive MaterialsDue to the unique chemical properties of 8-HQ and its derivatives many of these compounds nowadays have application in agricultural and medical fields. 8-HQ and numerous of its derivatives exhibit potent activities against several strains of insects, fungi and bacteria which make them good candidates for the treatment of many parasitic and microbial infection diseases. In addition, other 8-HQ derivatives have showedantitumor and antioxidant activities.
Fungicidaland Insecticides Properties of 8-HQ Derivatives8-HQ 1 is a potent lipophilic metal chelator whose 8-hydroxyquinoline copper chelate, CuQ12,is used extensively as a fungicide in many countries to control the diseases of freckle, scab, and black spot in cucumber, grape, wax apple, pear, and citrus [9]. Mono-chloro- and mono-bromo-substituted 8-HQ at positions 2-, 3-, 4-, 5-, 6-, and 713 showed antifungal activity against Aspergillus niger, Aspergillus oryzae, Myrothecium verrucaria, Trichoderma viride and Trichophyton mentagrophytes [10]. The 5,7-dichloro and 5,7-dibromoderivatives 14 were the most fungitoxic of the compounds tested (Figure-4).
High insecticidal effects were found for 8-hydroxy-2-methylquinoline15 against Laodelphax striatellus, Nilaparva talugens, and Sogatella furcifera which suggest that these compounds may be useful as new preventative agents the damage caused by a wide range of pests in rice farming areas [11].The 7-chloro and 7- bromo-5-sulfonic acids 16 and the 5-chloro and 5-bromo-7-sulfonic acids showed fungal inhibition within one order of magnitude of that of 8-quinolinol. It is suggested that a non chelating mechanism is in part responsible for this fungi toxicity(Figure-5) [12].
AntibacterialProperties of 8-HQ DerivativesThe antibacterial activity of 8-hydroxyquinoline and its derivatives is long-known. The drugs from this group are used as chemotherapeutics in medicine for more than 120 years. Some newly synthesized derivatives of 8-HQ were shown to exhibit a higher microbiological activity. In this regard, 8-hydroxyquinoline1 has bactericidal activity of comparable potency against non-replicating and replicating Mycobacterium tuberculosis (Mtb) and Staphylococcus aureus, a property not observed for anti-infective agents currently approved for treatment of tuberculosis [13,14]. In addition, 5-alkoxymethyl-8-quinolinolsuch as compound 17(Figure-6) exhibits greater activity against bacterial strains and fungal strains than do 8-HQ 1,but moderate activity compared with standard drugs [15].The substitution of phenyl rings, however, does not have more effect on the fungicidal activity. Another 8-HQ derivative that exhibits antimicrobial activity is 7-Morpholinomethyl-8-hydroxyquinoline18(Figure-6).Compound 18 was found to be more active against Gram positive bacteria than Gram negative bacteria and its potency correlated with its iron chelation. For this reason, a chelate between 18and Fe in a ratio of 2: 1 exhibited greater antibacterial activity than 18alone. Among the organisms tested, Micrococcus flavus was most susceptible with a MIC of 3.9 μg/ml [16]. It is suggested that MO-8HQ exerts its biological activity as a membrane-active agent through metal ion chelation.5-nitro-7-((4-phenylpiperazine-1-yl-)methyl)quinolin-8-ol 19(Figure-6) was found to be a putative inhibitor of type III secretion (T3S) in the Gram-negative pathogen Yersinia pseudo tuberculosis. Compound 19targets both the extracellular bacterium Y. pseudo tuberculosis and the intracellular pathogen Chlamydia trachomatis in cell-based infection models [17].
Antitumor and Anti HIV Activities of 8-HQ DerivativesTris (8-quinolinolato) gallium(III) 20 showed 10-fold greater potent inhibitory effects against A549 human malignant lung adeno carcinoma cells than did GaCl3 [18].Iron chelation in tumoral cells has been reported as potentially useful during antitumor treatment. In this regard, quilamines 21 are a new generation of iron chelators with a structural design based on 8-HQ scaffold linked to linear polyamine vectors. These new 8-HQ derivatives have been demonstrated to display an efficient antiproliferative activity in the micromolar range. In addition, cytotoxicity was only observed at concentrations higher than100 μM [19]. The 8-HQ glucoconjugate,8-quinolinyl-β-Dglucopyranoside 22, has been shown to be cleaved in vitro by β-glucosidase and exhibit antiproliferative activity against different tumor cell lines in the presence of copper(II) ions [20]. Poly hydroxylatedstyryl quinolines (SQLs), such as compound 23, are potent HIV-1 integrase (IN) inhibitors that block the replication of HIV-1 in cell culture at nontoxic concentrations as revealed by in vitro experiments [21]. The structural requirements for biological activity are a carboxyl group at C-7, a hydroxyl group at C-8 in the quinolone subunit, and an ancillary phenyl ring.
8-HQ Derivatives as Anti-Neuro degenerative AgentsAlthough neuro-degenerative diseases like Parkinson’s, Alzeheimer’s, Huntington’s and Prion’s have complex pathogenesis, scientists belief that radicals, trace metals and life style are generally accepted reasonsfor their progress and complications. Alzheimer’s disease (AD) is a fatal neurodegenerative disorder that primarily affects the elderly, presenting clinically as progressive memory loss and behavioural abnormalities. Most of the therapeutic strategies to treat AD work by targeting amyloid-β peptide, a small amyloidogenic peptide that accumulates in the AD-affected brain [22].These treatments include inhibitors of Aβ production, anti-Aβ immunotherapy, and therapeutics that promote Aβ clearance. However, most of these treatments have eventually failed to slow the progression of the disease. An alternative AD treatment strategy is to target the interaction between Aβ and metal ions using compounds that act as zinc, copper, and iron chelators [23]. All the three metals are involved in the deposition and stabilization of amyloid plaques in the brain. The first 8-HQ derivative that has been tested in vivo was 5-chloro-7-iodo-8-hydroxyquinoline (clioquinol, CQ, 24, figure-8) because of its selectivity as a chelator of Zn2+ and Cu2+ and its ability to cross the blood-brain barrier [24].In transgenic mice with Alzheimer symptoms has led to a 49% decrease in brain Aβ deposition and an improvement of symptoms [25]. Unfortunately, the further development of CQ as a potential drug for AD treatment was not progressed due to small contamination of diiodo-8- hydroxyquinoline, a known carcinogen, occurred during the larger scale production of CQ. Recently, a novel 8-HQ derivative, PBT2, is adopted for further clinical development as a therapeutic for AD instead of CQ [26].Moreover, Preclinical experiments of 5-[N-methyl-N-propargylaminomethyl]-8-hydroxyquinoline 25have shown activity against Alzheimer’s disease (Figure-8) [27].
8-Hq Derivatives In Oleds ApplicationsIt has been almost more than two decades since tris(8-hydroxyquinoline) aluminium (III) [AlQ3, 26, figure- 9]was introduced as an emitter and electron-transporting material in organic light-emitting diodes (OLEDs) by Tang and Van Slyke [28]. Nowadays, OLEDs are gradually replacing liquid crystal displays (LCD) as the most important fullcolor flat panel display technology.AlQ3 is considered a popular molecule in OLED industry because of its thermal and chemical stability. In order to improve the efficiency of MQ3(M = metal ion) complexes, researchers have centered their efforts on two major aspects: altering the chelated metal ion and modifying the structure of the chelating ligands.
Effect of CationsCations such as Al(III),Bi(III),Rh(III),Pt(II),Be(II), Mg(II), and Zn(II)were each investigated as a possible chelating metal ion in OLEDs [29-31].The heavy-metal complexes [M = Pt(II), Pb(II), and Bi(III), exhibit long-lived phosphorescence (τ ≈ 2-4 μs) and excited-state absorption (ESA) in fluid solution. Diaza-18-crown-6 hydroxyquinoline derivatives (DCHQ, 27, figure-9) bind Mg(II) with much higher affinity than other available metal ions and show a strong fluorescence increase upon binding [32].
Effect of LigandsIt has been predicted that electron-donating substituents in the C-5 or C-7 position of the quinoline skeleton cause a red-shift of the complex emission, while a blue-shift is expected when electron-withdrawing groups are attached in the same positions. In 2006, Anzenbacheret al prepared series of 5-substituted tris(8-quinolinolate) aluminum(III) complexes bearing aryl 28 or aryl ethynyl 29moieties (Figure-9) in order to explore the possibility of tuning the emission to obtain blue-, green-, and red-emitting materials [33]. These new prepared complexes exhibited successful tuning of the emission color covering the whole visible spectrum (λ = 450-800 nm) and their photophysical properties were found to correlate with the Hammet constant of the respective substituents. In addition, Sulimanet al demonstrated that Introduction of two electron-withdrawing substituted aryl groups at positions 5 and 7 of the quinoline skeleton in AlQ3complex30(Figure-9) causes simultaneous energy lowering of both HOMO and LUMO [7].
8-Hq Derivatives In Chemosensors ApplicationsRecently, a great number of researches are directed towards developing ion-selective fluorescent chemosensors for transition metal ions using 8-hydroxyquinoline and derivatives. Chemosensors are molecules that associate with a target analyte and provide a measurable response indicating of binding. Owing to their chelating ability toward a great number of metal cations, 8-hydroxyquinoline (8-HQ) and its derivatives have found many applications in fluorescent sensing of biological and environmentally important metal ions.
8-HQ Derivatives for Al DetectionAluminium(III) ion exists widely in the environment due to acid rain and human activities. Its toxicity to the environment and to humansis well documented [34]. Therefore, detection of Al3+ is crucial to control its concentration levels in the biosphere, hence its impact on human health.8-Hydroxyquinoline1 was used to detect Al3+ in soil extracts with fluorometric detection limit of ∼1 × 108M (0.3 ppb) Al [35]. Moderate selectivity for Al3+ over other metal ions with detection limit reaching <10-7M under weak acidic conditions was reported for 8- hydroxyquinoline-carbaldehyde Schiff-base31 (Figure-10) [36]. A novel fluorescent sensor 32 was prepared and used as an ion-selective chemo sensor for Al(III) viaintra-molecular excimer formation, from which a significant red shift along with an intensity enhancement of the excimer emission was observed in the presence of Al(III).
In summary, due to the unique chemical properties of 8-hydroxyquinoline and its derivatives many of these compounds nowadays have application in medical, OLEDs, agriculture, chemosensors, and metal extraction fields. This article reviewed different aspects of 8-HQ and its derivatives including their syntheses and applications.