ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Sri Devi M1, Vinothini K1, Veronica Shalini2, Krishnamoorthy Masilamani3, Dharini Sivakumar4 and Arjun Pandian4
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
This study aimed to elucidate of Leucas aspera, the plant has been widely used as traditionally an antipyretic and insecticide. Different solvent extracts of L. aspera using Chicken Chorioallantoic Membrane (CAM) assay. Methanol leaf extract showed the maximum angiosuppressive activity of 3±1 inhibition, concentration-dependently displayed a strong inhibition in the chick chorioallantoic membrane (CAM) angiogenesis. The results thus suggest that, the plant Leucas aspera should be considered as a useful source for designing drugs to cure antiangiogenesis.
Keywords |
| Antiangiogenesis, Leucas aspera, Chicken Chorioallantoic Membrane (CAM) |
I. INTRODUCTION |
| Cancer is one of the severe human diseases which cause increasing mortality every year in the world. Tumour growth and systemic metastasis are highly dependent on angiogenesis [1]. It is a process involved in growth of new capillaries from existing ones, which is a regular process that rarely occurs under normal conditions. Many diseases are driven by persistent unregulated angiogenesis [2]. Angiogenesis is a normal process in growth and development of blood vessels [3] and is a process essential for tumour growth. It is a tightly regulated process that involves signalling and extracellular matrix that induces the migration of endothelial cells to target the areas which are sources of proangiogenic signalling compounds [4]. Thus inhibition of angiogenesis is the prime target to afflict the growth of solid tumours. |
| Angiogenesis promotes growth of tumours by providing nutrients and oxygen, meanwhile facilitating tumour invasion and metastasis [5], hence a target for cancer chemotherapy. Antiangiogenic therapy aims to prevent the formation of new vessels around tumours, which remains in the dormant state until it can stimulate blood vessel growth from nearby pre-existing capillaries [6]. Thus the antiangiogenic therapy results in ablation of tumour vessels and prevents cancer which is more advantageous and is less toxic than chemotherapy agents [7]. Thus identifying antiangioenic pathway and synthesis of novel drug for therapeutic purpose are underway and natural products still represent an important source of leads for drug development. |
| Leucas aspera belongs to family Lamiaceae, an annual herb found throughout India as a common weed [8]. The juice of the leaves is used as remedies for cough [9], cold, psoriasis, chronic skin eruptions, chronic rheumatism, snake bits, poisonous insects, scorpion sting, used as insecticides and mosquito repellent in rural area [10]. The whole plant is traditionally used for analgesic, antipyretic, anti-rheumatic, anti-inflammatory, antibacterial, antifungal treatment and its paste is applied topically to inflamed areas [11], [12]. The antiangiogenic property of the plant has been studied using CAM assay. |
| Chick chorioallantoic membrane (CAM) assay has been widely used to study angiogenesis. It is performed in fertilized chicken eggs, which was first developed by Dr. Joseph Leighton, is one of the conventional histological procedures [13] associated with the proliferation of new vessels and tumour neovascularisation [14] by direct observation, where the number of blood vessels was counted [15]. |
| Literature survey of this plant has no reports for antiangiogenesis for which the present study was carried out for the plant to confirm the therapeutic value. Antiangiogenesis were carried out with solvent extracts of wild leaf and stem parts of the plant using Chorioallantoic Membrane (CAM) assay which is a part of our search for natural product based antiangiogenic agent. |
II. MATERIALS AND METHODS |
| A. Source of Plant Material |
| Fresh aerial parts of the Leucas asperra were procured from Jeppiaar Engineering College, Kanchipuram (D.T), Chennai. |
| B. Solvent Extraction |
| Fresh leaves and stem of the plant L. aspera were collected and washed with tap water followed with distilled water. These were shade dried and coarse powdered using mechanical methods and stored in airtight container. The airdried and powdered leaf and stem were macerated with Methanol, Chloroform, Acetone, Ethyl Acetate and Hexane at a concentration of 1g/100mL for 48 h. The suspension was then filtered through Whatmann filter paper (No. 1) and the solvent was removed at low temperature (40ºC-50°C) [16], [17]. |
| C. Antiangiogenesis – Preparation of Sample |
| Fifty eggs were procured from TNAUVAS Madhavaram, Chennai Seven day old fertilized brown leghorn eggs were used for this assay. Crude extracts (10 mg/mL) were dissolved in PBS solution. For ease of application, pellets of this solution 50μL/pellet, 100μL/pellet and 150μL/pellet were prepared and added drop wise on gelatine sponges and applied onto Chorioallantonic membrane (CAM). |
| D. Chorioallantoic membrane (CAM) assay |
| Seven days old fertilized brown leghorn eggs were collected from the hatchery and the eggs were cleaned with 70% Ethanol. A small window of 10 cm2 was made in the shell of the egg. Next, air was sucked out from the egg to bring their membrane down. Through the window of the each egg, sterile disc of gelatine sponge containing different concentrations of 50 μL, 100 μL and 150 μL of crude extract and its fraction were implanted inside the egg and at the junction of two blood vessels of the CAM subsequently. The opening was sealed with parafilm and the eggs were re incubated at 37°C in an incubator for 72 h. These windows were then reopened and inhibition of vessels formation were observed in terms of number and calibre and finally compared with PBS used as control. Each experiment was performed in triplicate [6]. |
| E. Statistical analysis |
| The data were subjected to One-way Analysis of Variance (ANOVA) to evaluate the significant of difference of means of various treatment groups using SPSS statistical software package (Version: 10). The values are presented as mean ± S.D and P<0.05. |
III. RESULTS AND DISCUSSION |
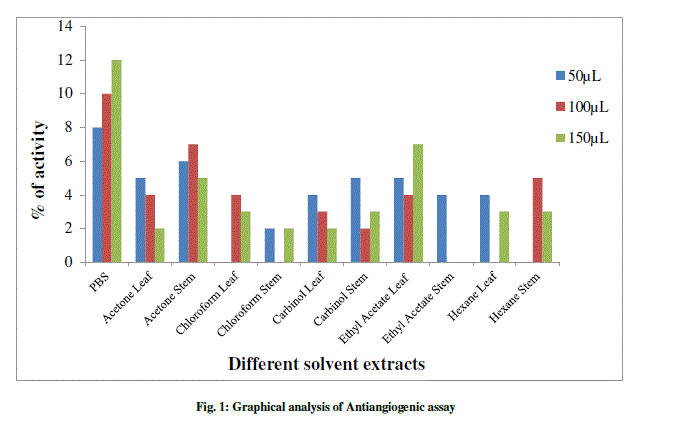
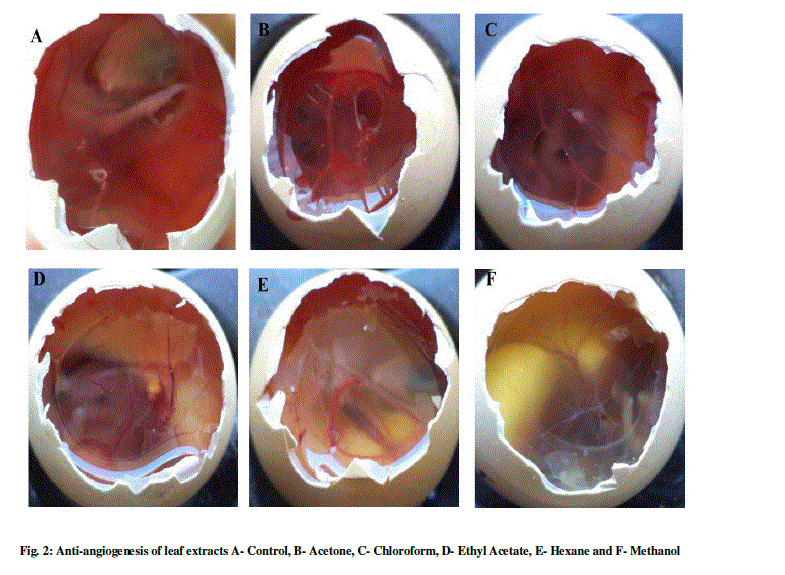
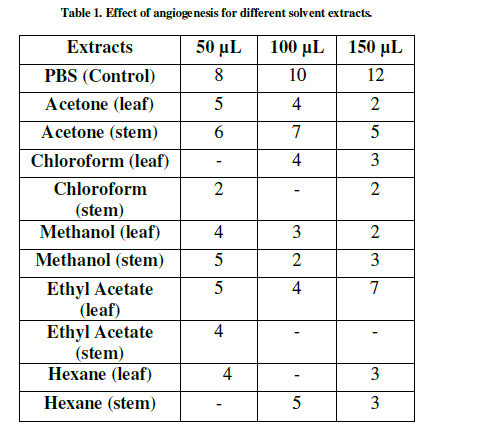
| The anti-angiogenesis activities of leaf and of L. aspera are shown in Table 1 and Figure 1-3. The search for plant-based anti-angiogenic agents is carried out using bioassay-guided fractionation wherein only the active extract and subsequent active fractions are pursued to minimize the efforts [18]. The methanol extracts of leaf and stem showed good inhibition followed by chloroform, acetone, hexane and ethyl acetate. This is supported by [19] who reported methanol extracts of Sphenocentrum Jollyanum exhibited good anti-angiogenic activity and acetone extracts showing angiogenic property for Boerhaavia diffusa [20]. The ethyl acetate extracts of Ardisia pyramidalis showed a better inhibitory effect [21]. |
 |
 |
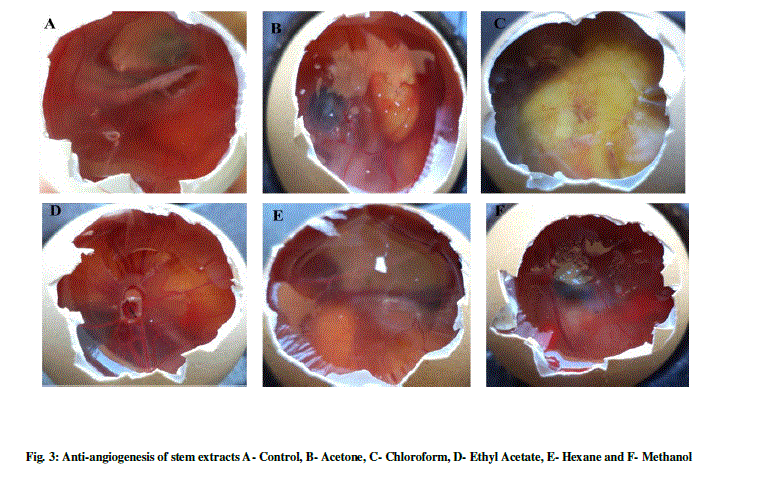 |
| Thus the CAM assay proved to be one of the better options for carrying out anti-angiogenesis. This has been reported by a number of researchers [22]-[24]. There has been a report by using transgenic zebra fish for curcumin micelles [1]. The wild plant extracts were better inhibitors, thus making them more reliable. This was in concordant with reports of [25], who studied the anti-angiogenic activity for Aloe vera gel extracts and also [2], who reported activity for different Chinese medicinal herbs. |
| There are a number of methods for determining the angiogenic activity. It is apparent that no single angiogenic assay can elucidate the entire process of angiogenesis whereas these assays are useful in combinations. Many technological advances have occurred recently in angiogenesis and quantification of newly formed micro vessels in laboratory animals that mimic various human diseases [26]. |
 |
IV. CONCLUSION |
| To conclude, the anti-angiogenic property was best exhibited for the wild plant extracts of methanol, followed by chloroform and acetone. The results showed that the inhibition of angiogenesis by the extracts is due to the suppression of vascular endothelial cell spreading, migration and angiogenesis. The search and discovery of novel antiangiogenesis are likely to be hope to the millions of sufferers of chronic disease. Since there have been no reports for anti-angiogenesis of L. aspera, this study would help for better research. This work suggests that this plant L. aspera should be considered as a useful source of material for human health as an antiangiogenic agent. |
References |
|