ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
| Shiv kumar verma1, Anand kumar2, Ashish3, Mira debnath (das)* Research scholar, School of Biochemical Engineering, Indian Institute of Technology (BHU), Varanasi, India |
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
The study was carried out to screen and isolate endophytic fungi, showing antimicrobial activity, from Root and stem tissues of Argemone maxicana (family papaveraceae). A total of fourteen Endophytic fungi, based on morphological characteristics, were isolated (assigned as AMS1- AMS8 for stem isolates and AMR9-AMR14 for root isolates). Among these fourteen isolates, AMS5 was found to show maximum antimicrobial activity, in compare to other isolates, against gram positive, gram negative bacteria and fungi. The antimicrobial activity was tested against Escherichia coli. Pseudomonas aeroginosa, Bacillus subtilis, Staphylococcus aureus, Ralstonia solanacearum, X.oryzae Penicillium chrysogenum, Candida albicans, Phoma exigua, Sclerotium rolfsii and Sclerotinia scleratiourum. Minimum inhibitory concentration (MIC) of crude extract against test microorganisms was determined. FTIR analysis of crude extract was carried out for functional group.
Keywords |
| Endophytic fungi, Antimicrobial activity, Minimum inhibitory concentration, FTIR |
INTRODUCTION |
| There are approximately 300,000 different plant species on our planet [1]. several hundred of these have been examined, and it is estimated that there are over one million endophytic fungi ,in association of many plants ,exist in nature [2] The success for naturally occurring therapeutic agents depends on fractionation and purification procedures. Technique of careful fractionation of the culture broth and mycelium extract lead to the isolation of the metabolite responsible for the antibacterial activity. In this context results of antibacterial testing of crude extracts and purified substances have been obtained from different endophytic fungi. In a more recent study it has been reported that antimicrobial activity of crude extracts tested a total of 385 extracts from 150 fungal entophytes with an antimicrobial screening test from mangrove fungal endophytes [3]. Two new antimicrobial compounds, 10-oxo-10H-phenaleno[1,2,3-de]chromene-2-carboxylic acids, xanalteric acids I and II and 11 known secondary metabolites were obtained from extracts of the endophytic fungus Alternaria sp., isolated from the mangrove plant Sonneratia alba collected in China These metabolites showed activity against E. faecalis, Pseudomonas aeroginosa. S. epidermidis [4] Search for antimicrobial metabolites is continuing because pathogenic organisms are showing resistance against available antimicrobial agents [5]. As a consequence, natural products research is always hunting for antimicrobial compounds which are the starting compounds for many more synthetic organic chemistry. In general, many antimicrobial compounds are found in plants but use of plants for extracting these compounds on large scale would cause them to be diminished rapidly. Therefore alternative approach apart from chemical synthesis should be sought by targeting endophytic fungi one of the most suitable resources for this purpose. Endophytytic fungi exist mainly in roots, leaves, and stems of living tissues of different plants, establishing mutual relationship without showing in fact any symptom of diseases [6]. The endophytic fungi were distributed based on the ecological and physiological factors in plants such as geographical location, age and specificity of host tissue [7]. Endophytic fungi inhabit millions of distinctive ecological niches in many abnormal environments [8]. Temperate plants are maximally studied for endophytic Fungi [9]. Endophytic fungi have been widely investigated as source of variety of bioactive compounds [10]. Bioactive compounds show interesting and attractive properties such as antibacterial [11], antifungal [12], anticancer [13], antiprotozoal [14], antioxidant [15], antiviral [16], antimalarial [17], antitubercular [18], immunosuppressive, antidiabetic and antiviral [19]. These bioactive compounds could be mainly classified as Alkaloids, steroids, terpenoids, quinones, phenylpropanoids, isocoumarins, lignans, phenols and lactones etc [20]. These bioactive compounds used for the healthcare purpose for human beings [21]. Several natural products produced by endophytic fungi have unique structures and large bioactivities which applied in agricultural, industrial and medicinal fields [22, 23]. Approaches for development of new antibiotics have been pursued, such as combinatory chemistry tools but only a few broad spectrum antibiotics are reported to be produced by the pharmaceutical industry at the present time [24]. |
| In our present work, we have isolated endophytic fungi associated with Argemone maxicana and screened them for their antimicrobial potentials against human and plant pathogenic bacteria and fungi. The plant which was chosen as study material is diuretic, purgative and also used to eliminate worms. It is useful in treatment of leprosy, skindiseases. Anti-inflammatory properties of plant extract have also been reported. Roots extract is antihelminthic. Juice is used to cure ophthalmia and opacity of cornea. Seeds are purgative and sedative. Seeds resemble mustard seeds and in India it is used to adulterate mustard seed. Seed yield non edible toxic oil and which is responsible for fatal dropsy when used with mustard oil for cooking. In Homoeopathic system of medicine, the drug prepared from this herb is used to treat the problem caused by tape-worm. |
MATERIALS AND METHODS |
2.1 Plant material |
| Plant material was collected from road side of Indian institute of technology, Banaras Hindu University (BHU), Varanasi (25.5-N 82.9-E, elevation 279 ft/85 m), India, in March 2013. The plant was identified on the basis of external morphology characteristic features. A complete mature and healthy plant was rooted out from soil surface. The samples were collected in sterile polythene bags and brought to the laboratory in an icebox. Samples were preserved at 4 0C in a refrigerator and processed for isolation of endophytic fungi immediately. |
2.2 Isolation of endophytic fungi |
| To eliminate epiphytic microorganisms, Root and stem samples of Argemone maxicana were washed thoroughly in running tap water for 15 min followed by four times washing with double distilled water to decrease the concentration of microorganisms from the sample surface. The surface treatment was done adopting the methodology of Petrini et al. (1992), and the effectiveness of surface sterilization was checked following to the method of Schulz et al [25]. |
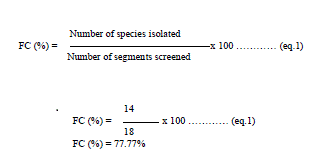
| To remove surface microorganisms, stem and root were dipped in 70% ethanol for 2–3 min and in aqueous solution of sodium hypochlorite (4%) for 3–4 min and then washed with 70% ethanol for 40s. The tissues were then rinsed three times with sterile double distilled water and allowed to surface dry in sterile conditions. The samples of root and tissue were dissected with sterile knife, into small pieces of 5x5 cm2. To confirm complete sterilization a small volume of last washing water was inoculated into petri dishes containing peptone 5 (g/l), malt extract 5 (g/l), sodium chloride 1.0(g/l) and agar 2.5%, pH of the medium was adjusted to 5.5. After surface sterilization, roots were cleaved aseptically into small pieces of 0.5 cm length and aseptically transferred to sterilized petri dishes containing (g/L): yeast extract 3.0, malt extract 3.0 peptone 5.0, dextrose 10 and agar 2.5%. The pH of medium was adjusted to 5.6.To check bacterial growth inoculation medium was supplemented with streptomycin (150 μg/mL) and chloramphenicol (150 μg/mL). The petri dishes were incubated at 28°C until the outcomes of endophytes were discerned. Hyphal tips originating from segments were transferred to Petri dishes containing PDA medium devoid of antibiotics. Each isolate was then grown and examined to make certain that it was originated from a single organism. All fungi present were isolated; sub cultured, and kept on PDA for further Identification. The frequency of colonization (FC %) was calculated taking into account the number of strains isolated (Ni) and the total number of fragments (Nf) [23]. |
 |
2.3 Fermentation conditions |
| All 14 fungal isolates were cultured for large scale production of antimicrobial metabolites. A piece of agar medium (0.5 × 0.5 cm) containing fungal hyphae was inoculated in each 250-mL Erlenmeyer flask containing100 mL sterile potato dextrose broth (PDB) medium, autoclaved at 125 âÃâæC for 15 min., and incubated on a rotary shaker at 145 rpm and 28 ºC for 8 days. After fermentation is completed, the culture was filtered through three layers of muslin cloth to remove the mycelia. Then the culture filtrate was extracted thrice with ethylacetate. Then culture filtrate become divisible into aqueous and solvent phase. The solvent phase and aqueous was separately reduced under pressure using rotary vaccum evaporator at 350C and 50 0C respectively. The residues were redissolved in dimethyl sulphoxide (DMSO) for subsequent spectrometric analysis. |
2.4 Antimicrobial activity assay |
| Antimicrobial activity of the crude extract of all 14 isolates was tested by the disk diffusion method[22] against six human pathogenic bacteria (Escherichia coli, Bacillus subtilis, Staphylococcus aureus, Pseudomonas aeroginosa, two plant pathogenic bacteria; Ralstonia solanacearum Xanthomonas oryzae and human pathogenic fungus Candida albicans and four plant pathogenic fungi sclerotium rolfsii, Sclerotinia scleratiourum and penicillin sp . Human pathogenic bacteria and fungus was purchased from the Institute of Microbial Technology (IMTECH), Chandigarh, India. Plant pathogenic fungi and bacteria were taken from Institute of Agriculture Science, BHU Varanasi. Nutrient agar plates were inoculated with an overnight culture of each bacterial suspension. in the same way, for the fungal pathogens, PDA plates were inoculated with each fungal suspension. The inoculated organisms were evenly spread out with sterile glass spreader. The plates were then incubated at 36±1 °C for 24 and 48 h, respectively, for bacteria and fungal pathogens. The zone of inhibition was recorded after the specified incubation period (24 hr). Three replicates were maintained in each case. |
2.5 Minimum Inhibitory Concentration (MIC) |
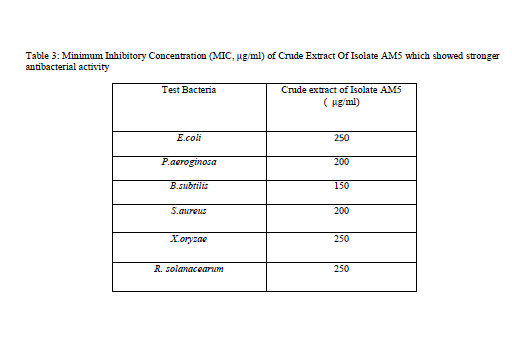
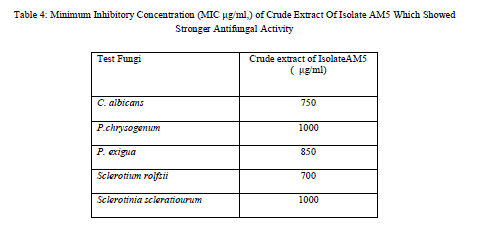
| MIC of crude extract from isolate AMS5 that showed inhibitory effect was determined by dilution methods [27]. The MIC values of crude extract from isolate AMS5 is shown in Table 3. The result indicated that fungal crude extract clearly showed antibacterial property against gram-positive and gram-negatives bacteria. The antibacterial activity of bioactive compound produced by isolates RMS5 is comparable with chloramphenicol as standard antibiotic. The extract obtained from isolate RMS5 had MIC values, 250 μg/ml for E.coli, Ralstonia solanacearum and X.oryzae. MIC values, 200μg/ml was observed for P. aeroginosa, S. aureus and 150 μg/ml for B. subtilis. Similarly MIC value for test fungi was determined, the extract in this case had MIC value of 750 μg/ml, 1000 μg/ml, 850 μg/ml,700 μg/ml and 1000 μg/ml for C. albicans, Penicillium sp. P. exigua, Sclerotium rolfsii and Sclerotinia scleratiourum respectively.This RMS5 isolate could be excellent candidate for advance studies of their antibacterial bioactive compounds. |
2.6 FTIR Analysis |
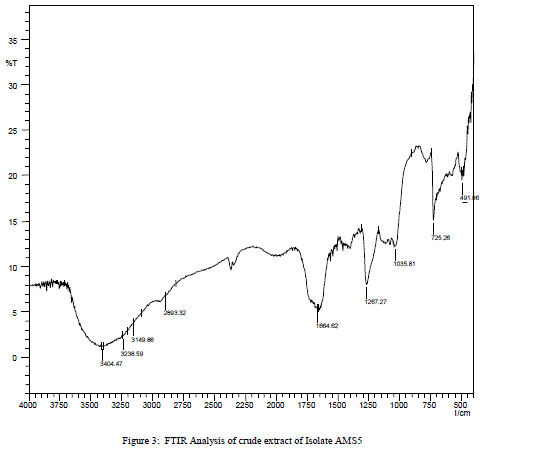
| The bioactive crude was subjected to FT-IR spectral analysis which showed the characteristic stretching frequency at 3238.59 cm-1, which indicates the presence of secondary amine, –NH. Stretching frequency at 3149.96 cm-1 and 3404.47 cm-1 correspond to phenolic group (–OH). A strong stretching was observed at 725.26 cm-1showing presence of mono substituted benzene ring. Another sharp stretching was observed at 1264.27 cm-1 indicating presence of acyl group. Presence of conjugated, aromatic and six atom ring was confirmed by more informative stretching at 1664.62 cm-1. Stretching at1035.81 cm-1 indicates possibility of presence of primary amine. Four minor stretchings were observed between 1450 to 1550 cm-1 showing presence of C-H (two stretching frequency), N-O and C-C bond. |
Results and Discussion |
| In this study a total of 14 fungi, based on morphology characteristics, were isolated from18 segments of root and stem parts of the plant (Argemone maxicana), the colonization frequency was calculated from equation 1 i.e. |
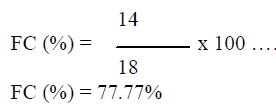 |
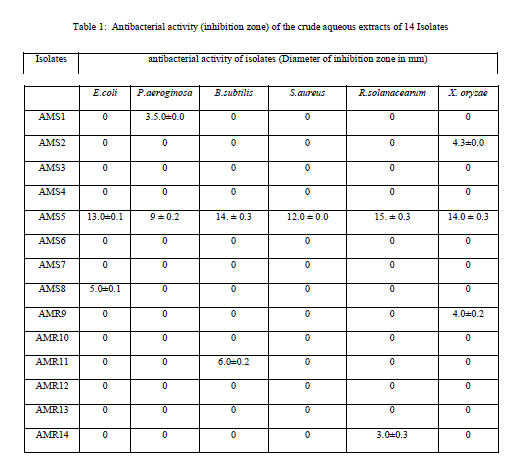
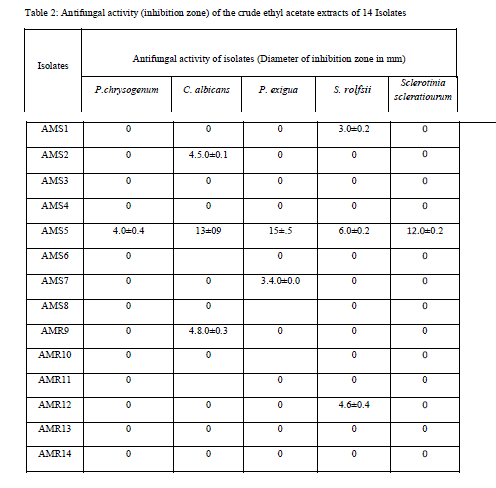
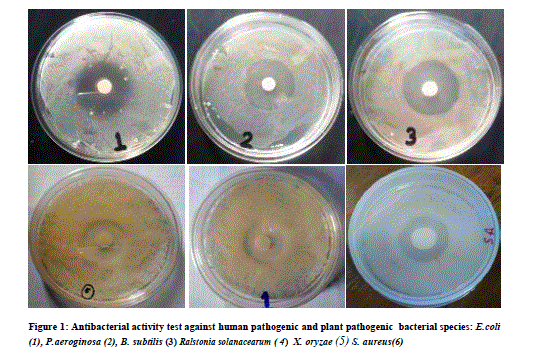
| 3.5 liter of fermentation was carried out for each fungal isolate. Volume of Aqueous filtered broth of each isolate was reduced, in vacuum oven, to make solid residue. Antimicrobial activity test was performed using disk diffusion method [25]. The disk was placed onto overnight cultured petridishes and loaded with the 10mg/ml concentrated crude metabolite for the testing antimicrobial activity against different microorganisms. Antimicrobial activity was tested against six bacterial cultures i.e., Escherichia coli. Pseudomonas aeroginosa, Bacillus subtilis, Staphylococcus aureus and Xanthomonas oryzae. Similarly antifungal activity was performed against five fungal species i.e., Penicillium sp., Candida albicans, Phoma exigua, Sclerotium rolfsii and Sclerotinia scleratiourum. Tested microorganisms were cultured on agar solidified medium. 20 μl crude extracts from all 14 isolates were applied on the disk (6 mm diameter). The bacterial cultures were placed in an incubator at 37 0C and fungal culture at 290C for overnight incubation. Inhibition zone was observed and diameter of inhibition zone was measured. Among all 14 isolates, AM5, from stem tissue was found to show prominent antimicrobial activity both against human pathogenic bacteria and human pathogenic fungus (Candida albicans) tested. The antifungal activity shown against plant fungi was stronger than bacterial counterpart. Xanthomonas, oryzae causes bacterial blight (BB) of rice which is one of the most important diseases of rice in most of the rice growing countries. Bacterial blight of rice has high epidemic potential and is destructive to high-yielding cultivars (cerials) in both temperate and tropical regions. Ralstonia solanacearum infects Potato (Solanum tuberosum); Banana, (Musa spp); Geranium (common name) (Pelargonium); Ginger (Zingiber officinale); Tobacco (Nicotiana tabacum); Sweet pepper (Capsicum spp); Olive (Olea europea),Tomato (Lycopersicum esculentum); Aubergine (egg plant) (Solanum melongena);). Therefore metabolites from this fungal isolate could be an important chemical remedy in checking loss of crops caused by these bacteria. Similarly, fungi which were tested are highly plant pathogenic fungi. Sclerotium rolfsii, an omnivorous, soil borne fungal pathogen, causes disease on a wide range of agricultural and horticultural crops, Phoma exigua is a fungal plant pathogen. It causes wet weather blight in cotton resulting in huge loss of cotton production. |
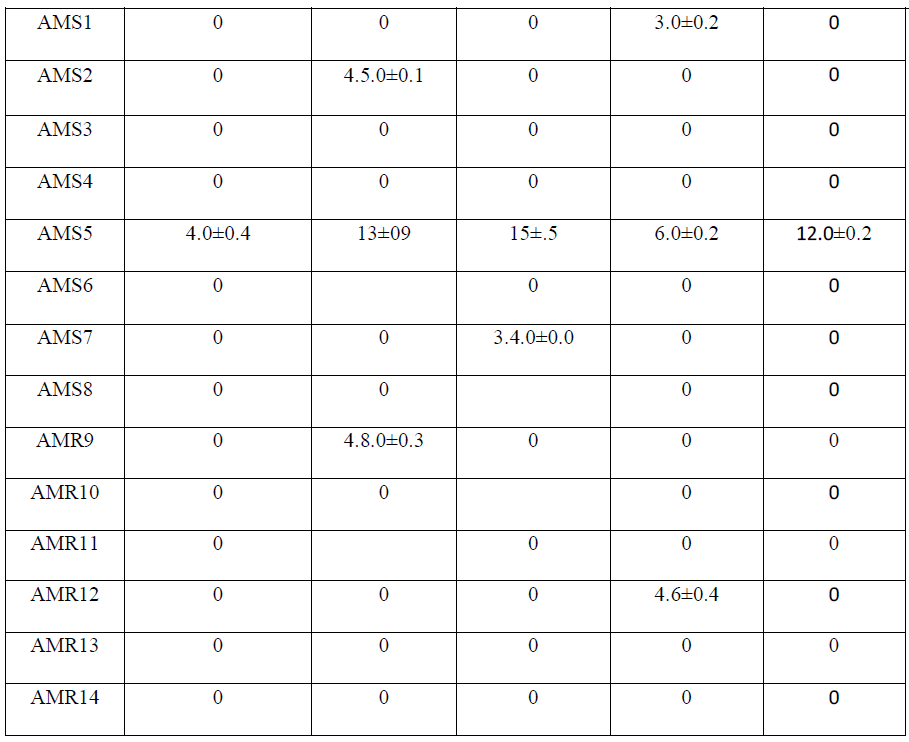
| Isolate AMS1, AMS2 AMS7, AMS8, AMS9 AMS11 and AMR14 showed antibacterial activity against at least one bacterium while AMS3, AMS4 AMS6 ,AMR10 AMR12 and AMR13 did not show any antibacterial activity and the results are listed in Table 1.Similarly, when these isolates tested for antifungal activity, AMS1, AMS2, AMS7, AMS9 and AMS12 showed antifungal activity, against at least one fungal species. AMS3, AMS4, AMS6, AMS8, AMR10, AMS11, AMR13 and AMR14 did not show any antifungal activity and the results are listed in Table 2. The MIC value (Table 3 and Table 4) of crude extracts for both bacterial and fungal species explained that the molecules present in crude extract could be a good source of antimicrobial metabolites which can be effectively applied against human pathogenic and plant pathogenic micro organisms therefore further work could be done to know its properties and chatacteristics. Different stretching frequency obtained on the basis of FTIR analysis, explained that the compounds in the crude extract more possibly may belong to alkaloid or terpenoid group of compounds. Stretching frequency at 1664.62 cm-1, confirmed the presence of conjugated, aromatic and six atom ring which gives information about basic ring structure system of alkaloid and terpenoid structure. Further investigation of bioactive metabolites produced by isolate AM5 is very necessary to be characterized using advanced analytical chemistry tools since the isolate obtained in this study is a prospective source for searching novel bioactive metabolites with an effective antifungal property against plant fungal pathogens. Taken together, this experimental study emphasized the assumption that endophytes of medicinal plants could be a hopeful source of antimicrobial substances. |
CONCLUSIONS |
| In this study preliminary screening of endophytic fungi revealed their potential to yield bioactive compounds which may play as a role of leading biomolecules for drug designing in pharmaceuticals industry. Compounds in purified form may be used as potential antifungal agents against plant pathogenic fungi. On the basis of the information obtained from FTIR analysis, it is concluded that mixture of compounds present in crude could be of alkaloid in nature. Further for growing of fungus in large scale, modification of culture conditions like varying composition if growth media, varying pH, and supplying some stimulants might help in getting improved production of the particular bioactive metabolites. |
| Endophytic fungi may be a vast source of bioactive metabolites, which can be used in treatment of various microbial infections and also provide research area for further investigation. But there are still some works remained to be done is the mechanisms of action of these antimicrobial compounds, the physiological and ecological roles of this fungus. Efficient strategies should be made for increasing metabolite content and yield of the fungal culture that necessitates to be further clarified and determined further studies are now needed to identify and characterize bioactive metabolites produced using advanced analytical chemistry and to explore more about applications of bioactive metabolites of endophytic fungi. |
| Table 1: Antibacterial activity (inhibition zone) of the crude aqueous extracts of 14 Isolates |
 |
 |
 |
 |
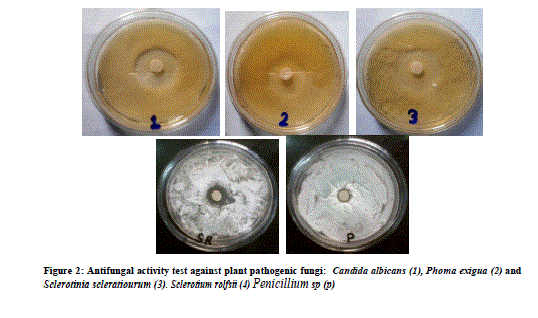 |
| Figure 2: Antifungal activity test against plant pathogenic fungi: Candida albicans (1), Phoma exigua (2) and Sclerotinia scleratiourum (3). Sclerotium rolfsii (4) Penicillium sp (p) |
| Table 3: Minimum Inhibitory Concentration (MIC, μg/ml) of Crude Extract Of Isolate AM5 which showed stronger antibacterial activity |
 |
| Table 4: Minimum Inhibitory Concentration (MIC μg/ml,) of Crude Extract Of Isolate AM5 Which Showed Stronger Antifungal Activity |
 |
 |
References |
|