ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Ramanibai Ravichandran1, Deepika Thangaraj2 and Madhavarani Alwarsamy3
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
To establish the larvicidal activity of solvent extract: Acetone, Methanol, Ethanol, Ethyl acetate, and Chloroform of two plants Neem (Melia azedarach) and Papaya (Carica papaya). L against Culex quinquefasciatus. The result was indicated that Acetone extract of both plants showed highest morality rate when compared with rest of the solvents. Thus C.papaya extract showed highest mortality rate of 61.6% mortality in 24hr at 500 ppm and 93.3% in 48 hr at 300 ppm. LC50 and LC90 values was found to be 80.56ppm and 380.67ppm, 60.89 ppm and 150.75ppm respectively. M. azedarach extract showed highest mortality rate of 90% at 500 ppm in 24hrs and 93.3% in 48hrs at 500 ppm. LC50 and LC90 value was found to be 305.20 ppm and 756.23 ppm, 179.29 and 450.09 ppm respectively. Secondary metabolites present in both plants was analyzed. The phytochemical analysis showed that different plants of Carica papaya and Melia azedarach was contain different bioactive compounds in varying proportions. The plant was extracted using five different organic solvent when compared with two plants, the Melia azedarach shows maximum result of bioactive components in methanol extract and shows the highest secondary metabolites.
Keywords |
| Melia azedarach, Carica papaya, Culex quinquefasciatus, larvicidal activity, phytochemical analysis. |
INTRODUCTION |
| Mosquito transmits more diseases than any other group of arthropods and reported to affect millions of people throughout the world (Anupam Ghosh et al., 2012). WHO has declared that the mosquito as “public enemy number one” because the mosquito is the principal vector of many of the vector-borne diseases affecting human beings and other animals. Mosquitoes constitute a major public health problem as vector of serious human diseases (Hag et al., 1999) comprising approximately 3,500 species of mosquitoes are grouped into 41 genera and found beyond the tropical and sub tropical region of the world with they are classically associated . Several species belong to genera Aedes , Anopheles and Culex vectors for the pathogen of various diseases like Anopheles -malaria Aedes -yellow fever, dengue, chikungunya and Culex -West Nile, Japanese encephalitis filariasis (White et al., 2004). Female mosquitoes suck blood from vertebrates to obtain the necessary nutrition to lay their eggs in turn injected the pathogens in to the host animal (Reiter et al., 2001). While many mosquitoes are distinctly selective feeders, restricted to one or few closely related species but some may feed in a less restrictive manner varying between mammals, birds and reptiles. Mosquitoes which regularly feed on humans and in which pathogens can complete an obligatory life cycle phase and multiply in the mosquito’s salivary glands can be important vectors of human diseases. Mosquitoes breed in water occasionally depositing eggs directly on water but generally prefer a variety of moist surfaces such as tree holes and containers. Human activities such as the production of a large amount of the environmental debris that holds water pools including disposable bottles, cans , discarded tires and storage of water in and around living premises. When reliable piped home water supplies are unavailable or unreliable may markedly increase the available mosquito breeding sites (White et al., 2004). Integrated mosquito management approach should be adopted for getting an effective and efficient biological control on mosquito (Ali et al., 2010). The biological control does not cause chemical pollution to human as well as environments however it is specifically toxic to specific targeting species not affecting the non-target organisms (Bansal et al., 2012). Hence, it is considered as a better method for mosquito control by many people. The most common control method is predatory method. The essential feature of a mosquito predatory fish and aquatic bugs and beetles, tadpoles, copepods have been used as biological control agents against mosquito. However they are still in experimental stages of development or give limited effect on the control except the fishes. Although the guppy, Poecilia reticulate can tolerate moderate degree of pollution heavily polluted water is not suitable habitat for fishes. The botanical insecticides are Eco-friendly, environmentally safer and biodegradable the evaluation of plant extracts for their deleterious effects on insects is one of the approaches used for the search of novel botanical insecticides. Though many plants have been shown to possess insecticidal / larvicidal and growth inhibition activity against mosquitoes. The use of different parts of locally available plants and their various products in the control of mosquitoes has been well established. |
MATERIALS AND METHODS |
| 2.1 Collection of plant materials |
| The plant leaves of Melia Azedarach and Carica papaya were collected from keelarakollai at Kancheepuram district. The collected plant was identified at CAS in Botany University of Madras, Guindy Campus, Chennai-25. The plant leaves were washed with tap water and shade dried at room temperature (Periyanayagam et al., 2007). With the help of electrical blender the leaves were powdered. |
| 2.2 Preparation of leaf extract |
| 20 g of blender powder was soaked in 50 ml of the acetone and then kept for 24 hrs to dissolve the active components of plant leaf materials. The suspensions was latter filtered into conical flask using Whatman’s No.1 filter paper. The filtrates was put into the Gallenhamp vacuum oven to evaporate the extraction of solvent. Similarly four different organic solvent was used for preparation of extract (Chloroform, Ethanol, Ethyl acetate and Methanol). |
| 2.3 Preparation of stock solution |
| A stock emulsified water solution of the extract of 1000 ppm was prepared by using 1 gram of plant residue was dissolved in 5 ml of the acetone mixed well then it was dissolved in 95 ml of the distilled water (Sakthivadivel at al., 2012) .it was said to be 1% of stock solution(Jayapal subramaniam et al., 2012). likewise the ethanol and ethyl acetate extract was prepared with the help of 5 ml of acetone. but the chloroform and methanol residue could not dissolve completely in acetone so the Tween 20 (emulsifier ) one drop was added. |
| 2.4 Preparation of concentration |
| From the 1% stock solution 5 different concentration were prepared ranging from 100 ppm, 200 ppm 300 ppm 400 ppm 500 ppm respectively. |
| 2.5 Collection of larvae |
| The larvae of Culex quinquefasciatus was collected from Georgetown and transferred into tray then the larvae was separated based up on instars. The late3rd instar and early 4th instar was used for experiments. |
| 2.6 Larval bioassay |
| Bioassay on mosquito larvae was performed on late third and early fourth instars. The disposable plastic cups was taken and filled with 100 ml of tap water with different concentrations taken separately and mixed with water and in each cup 20 larvae was introduced . Each concentration had four replicate with appropriate control. The stock control was prepared by mixing 5 ml of acetone and 0.1 ml of Tween 20 in 100ml of tap water. The independent and combined toxicity observation were made to total number of larval mortality. The larval and pupal mortality were recorded after 24 hours and 48 hours . |
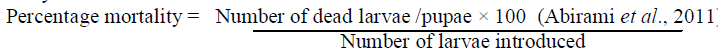 |
| 2.7 Statistical analysis |
| All data was subjected into average of larval mortality percentage with the help of statistical analysis and then the percentage of LC50 and LC 90 values were calculated using probit analysis (EPA Probit analysis program version 1.5) |
| 2.8 Qualitative Method of Phytochemicals |
| Qualitative analysis of Alkaloids (Mayer’s test - Evans, 1997),Carbohydrate (Fehlings test - Ramakrishnan et al., 1994), Proteins and aminoacids (Xanthoproteic Test), Flavonoids (Shinoda test), Steroids, Phytosteroids, Glycosides (Legals Test), Phenols (Ferric chloride test), Tannins (Ferric chloride test -Mace, 1963), Saponins (Foam test), Coumarins, Quinones has been carried out using the above references. |
RESULTS & DISCUSSION |
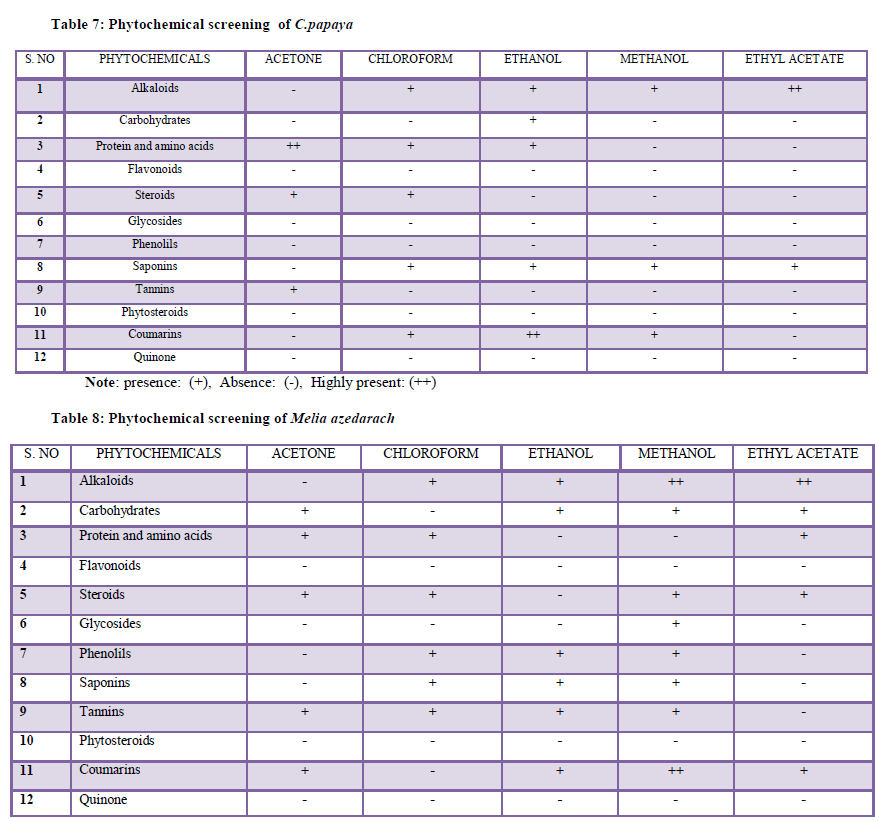
| Larval mortality of Culex quinquefasciatus after the treatment of five different solvents (Acetone, Chloroform, Ethanol, Ethylacetate and methanol) using the extract of C. papaya and M. azedarach leaf was observed. The results clearly evident that the larval mortality at different concentrations (100 to 500 ppm). |
| CARICA PAPAYA & MELIA AZEDARACH |
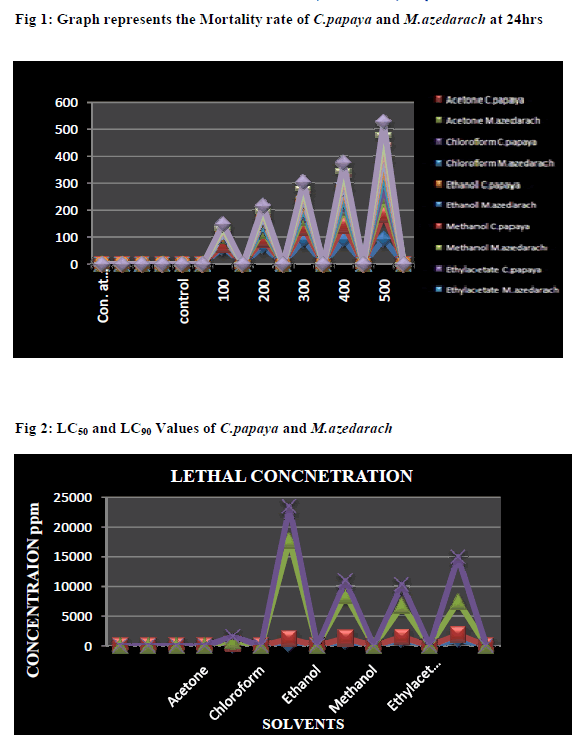
| The Figure (1-2) illustrated the Acetone extract of two plant leaves showed better results. 61.6% mortality was noted in C. papaya and 90% of 500 ppm at 24hrs was noted in M.azedrach. The LC50 and LC90 values of C. papaya was 80.56 ppm and 380.67 ppm and LC50 and LC90 value of M.azedrach is 305.20 ppm and 756.23 ppm respectively. The Chloroform extract of C.papaya was found to be 73.3% at 500 ppm and 35% at 500 ppm noted in M.azedrach. The LC50 and LC90 value of C.papaya was found to be 280.08 ppm and 1685.49 ppm and LC50 and LC90 value of M.azedrach was noted as 938.85 ppm and 5652.15 ppm. The Ethanol extract of C.papaya was found to be 43% and M.azedrach was found to be 45% at 500 ppm in 24hrs. The LC50 and LC90 value of C.papaya and M.azedrach was found to be 790.53, 7207.31 ppm and 564.95, 2393.51ppm. The Methanol extract of C.papaya was found to be 26.6% and M.azedrach was found to be 38.3% at 500 ppm in 24hrs. The LC50 and LC90 value of C.papaya and M.azedrach was found to be 1077.34, 5285.66 ppm and 439.12, 3478.52 ppm. The Ethylacetate extract of C.papaya was found to be 35% and M.azedrach was found to be 48.3% at 500 ppm in 24hrs. The LC50 and LC90 value of C.papaya and M.azedrach was found to be 1084.72, 5567.70 ppm and 809.02, 7487.65 ppm. |
| PHYTOCHEMICAL ANALYSIS |
| The present study was carried based on the secondary metabolites present in the plant leaves. The phytochemical analysis showed that different plants of Carica papaya and Melia azedarach are contained different bioactive compounds in varying proportions. The plant leaves were extracted using five different organic solvent. When compared to the two plant leaves Melia azedarach shows the maximum result of bioactive components, in this five solvent extract the methanol extract shows the highest secondary metabolites. However in this study the phytochemical screening of all solvent extract showed the presence of bioactive compounds which may retain a wide range of actions. The biological activity of the two different plant leaf extracts might be varied due to the different in their secondary metabolite composition. The most important compounds are chymopapain, papin which are found in C.papaya. This compound may jointly or independently contribute to produce the larvicidal activity against A.stephensi (Kovendan et al., 2012). The present study reported the toxic effects of the selected plant leaves against Culex mosquito larvae. The plant leaves of all the chemicals may be quite useful in increasing the efficacy of biological control agents because plant product produces a large variety of compounds. The larvicidal activities vary according to the plant species, plant part what we used, geographical location of the plant, photosensitivity of some of the compounds in the plant extract and the finally the solvent of extraction and the species responsible to the specific extract (Sukumar at al.,1991). Earlier reports revealed that the C.papaya ethanol leaf extract have been shown to have effective larvicidal activity against the I to IV instars of Culex quinquefasciatus larvae and pupae mortality rates. The LC50= I instar was 3.65 %, II instar was 4.28% III instar was 5.41% and IV instar was 6.70% and pupa was 7.50%. (Kovendan et al., 2012). reported that the acetone extract of Nirium indicum and Thenus orientalis have been studied having LC50 values of 200.87ppm, 127.53ppm, 209.00ppm, and 155.97ppm against C.quinquefasciatus and A.stephensi. respectively. |
| The methanolic leaf extract of Vitex negundo, Vitextrifolia, Vitex peduncularis, and Vitex altissima were used for larvicidal assay shown to have the LC50 values of 212.57ppm, 41.41ppm, 76.28ppm, and 128.04ppm, respectively against the early fourth-instar larvae of C.quinquefasciatus (Kannathasan et al., 2007). The current study of investigating the potential of C.papaya and M. azedarach leaves extract against C.quinquefasciatus proved the larvicidal efficacy of both the plants highly supported the previous literatures. However, the highest larval mortality was noted in Acetone extract of both the plants in which 100% mortality was found for C.papaya at 300ppm within 48hrs. In case of M.azedarach, the LC50 value was 60.86ppm and LC90 value was150.75 ppm respectively. It was clearly evident that the efficacy of plants was mainly due to the presence of secondary metabolites may responsible for the mortality of larvae. Roark (1947) described approximately 1,200 plant species, (Sukumar et al., 1991) listed and discussed 344 plant species that exhibited mosquitocidal activity (Shaalan et al., 2005). The current state of knowledge on larvicidal plant species and listed the phytochemicals, botanical ovicides, synergistic additive and antagonistic join action effects of botanical mixture against mosquito larvae. Several secondary metabolites such as steroids (Ghosh et al., 2008; Chowdhury et al., 2008; Rahuman et al., 2008; Zolotar et al., 2002), phenolics (Tripathi and Rathore, 2001),essential oils (Amer and Mehlhorn, 2006) were reported to have a remarkable mosquito larvicidal activity. Plant-derived substance have recently become of great interest due to their multiple application (Kavitha Sama et al., 2013). The medicinal plants are the richest bioresource of drugs of traditional systems of medicine (Sukanya et al., 2009). Even today plant materials continue to play a major role in primary health care as therapeutic remedies in many developing countries (August et at., 2008) |
 |
 |
CONCLUSION |
| The present study was carried out on the two leaf extracts of M.azedarach and C.papaya revealed the presence of medicinally active constituents. In this present study, the preliminary phytochemical screening of all extract showed the presence of bioactive compounds which may retain a wide range of actions. Among the two plants, the secondary metabolites was found rich in M.azedarach had shown the presence of alkaloids, carbohydrate, phenolics, steroids, aminoacids, glycosides, saponin, tannin, coumarin in all the extracts tested. The acetone extract of both C.papaya and M.azedarach showed the highest mortality rate than all other extracts. Interestingly leaves extract was found particularly rich in steroids. The presence of steroidal compounds would have effective larvicidal activity as cited in previous studies. High amount steroids metabolite may increase the high percentage mortality rate of Culex larvae. |
| From the observation conclude that some important specific secondary metabolites like steroids, Tannins, Coumarins, Proteins and aminoacids may contribute towards the larvicidal activity of mosquito larvae C. quinquefasciatus It was clearly proved that crude or partially purified plant extracts are less expensive and highly efficacious for control of mosquitoes (Jang et al., 2002; Cavalcanti et al., 2004). The plant extract are eco-friendly and are not toxic to vertebrates (Sharook et al., 1991). In this study, the result showed that the acetone extract of C.papya indicates the high percentage of blocking the development by induction of great mortality of larvae. Most of the epithelial cells degenerated and vacuolated after the treatment of 48h leaf extract of C.papaya. Histological changes were seen in the anterior and posterior regions of the midgut included separation in the epithelial cells from the basement membrane with damage of the peritrophic membrane. It was investigated that the mixing of the gut contents with haemolymph caused the larval mortality as reported by Al-Mehmadi et al.,(2010). |
ACKNOWLEDGMENT |
| One of the author (R. Ramanibai) gratefully acknowledged the financial support from University Grants Commission (UGC MRP 2013 ) and Ms. A. Kanimozhi for her lab assistances. |
References |
|