ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Mouna.H.M2., P. Jagan Mohan Reddy1, P.E Rajasekharan3, Ismail Shareef1, Sreekanth B4,
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Gymnema sylvestre (Retz.) R. Br. ex Sm., a rich source with gymnemic acid, has been used to treat diabetes since past 2000 years. A study was conducted to evaluate the genetic diversity of G.sylvestre collected from twelve different geographical locations of Karnataka state of India using ISSR molecular markers. A total of 80 clear and reproducible bands were amplified with different lengths using the 10 selected ISSR primers, of which 68 (85%) were polymorphic and remaining 12 (15%) were monomorphic. The high level of genetic variation (85%) observed among the populations of G.sylvestre implied the need to conserve populations to maintain within this species. As an important medicinal plant, it would be wise to save populations of G.sylvestre in different regions in order to limit population declines caused by over exploitation
Keywords |
| Anti-diabetic, genetic diversity, Gymnema sylvestre, gymnemic acid, gurmar, ISSR. |
INTRODUCTION |
| The use of traditional medicine and medicinal plants in most developing countries, as a normative basis for the maintenance of good health, has been widely observed (Tiwari and Madhusudana Rao, 2002). Since ancient times G. sylvestre is used to treat diabetes (Reddy et al. 2004).Gymnema sylvestre (Retz.) Schult. (Asclepiadaceae), a woody, vine-like climber, is a reputed traditional medicinal plant as a stomachic, diuretic and as a remedy to control diabetes mellitus and obesity (Sastri 1956; Porchezhian and Dobriyal 2003). Gymnemic acid has been identified as an active ingredient of G. sylvestre (Hooper 1987) predominantly found in leaves at the range of 10% of its dry weight (Khramov et al. 2008).The plant is distributed over most of India, China and Vietnam and popularly known as 'Gurmar' for its distinctive property of temporarily destroying the taste of sweetness. The total saponin fraction of the leaves, commonly known as `gymnemic acid', is shown to be able to inhibit glucose absorption in the small intestine and to suppress elevated glucose levels in blood (Yashioka, 1986; Suttisri et al., 1995; Shimizu et al., 1997). Since the gymnemic acid fraction is allegedly beneficial for treating diabetes and obesity, preparations containing Gymnema plants of Indian origin are now commercially available in Japan, Germany, and the USA as health foods. The molecular assessment of genetic diversity can also be directly useful in estimating the genetic relationships among the accessions. The development of random amplified polymorphic DNA (RAPD) markers (Williams et al, 1990) has proved useful for assessment of genetic variation. |
| Although a lot of chemical analyses have been performed on G.sylvestre, its genetic diversity is less characterized (Sahu et al., 1996; Liu et al., 2004; Daisy et al., 2009). Considerable biochemical and morphological variations have been reported in G.sylvestre (Thamburaj et al., 1996; Nair and Keshavachandran, 2006). The aim of the present study was to evaluate the genetic diversity of G.sylvestre collected from twelve different locations of Karnataka state of India using ISSR molecular markers. Characterization of genetic variations is helpful for understanding of intrinsic factors and variations responsible for production of various metabolites and better commercial exploitation of any economically important species. Molecular markers based on DNA sequences detect more polymorphism than morphological and protein-based markers and constitute a new generation of genetic markers (Tanksley et al. 1989). Among the various molecular marker techniques for detecting genetic diversity, Inter Simple Sequence Repeats (ISSR) is a powerful tool for investigating genetic variation within species (Zietkie wicz et al., 1994). |
II. MATERIALS AND METHODS |
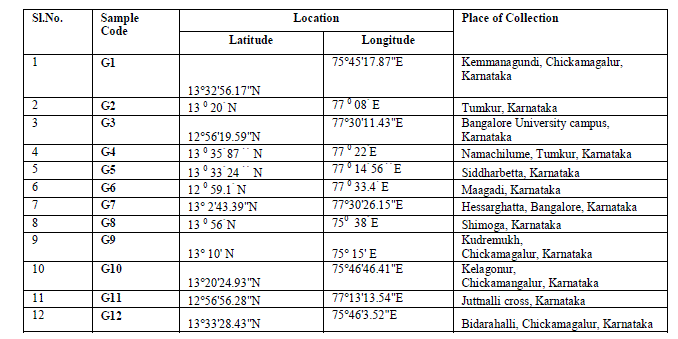
| Plant material: Twelve accessions of G.sylvestre were collected from different location of Karnataka state of India. The details and geographical distribution of the collected sample materials is shown in Table 1. The collected plants were established in Indian Institute of Horticultural Research, Bangalore field gene bank. |
 |
| DNA extraction: Total genomic DNA was isolated from fresh leaves using modified CTAB (Cetyl trimethyl ammonium bromide) method (Doyle and Doyle 1987). Quantity and quality of the DNA samples were estimated by comparing band intensities on a 0.8% agarose gel electrophoresis and by spectro photometeric method. |
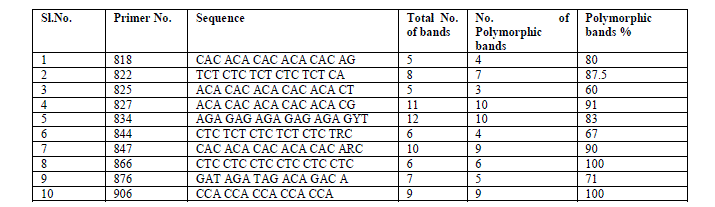
| ISSR analysis: The components of the ISSR-PCR were optimized with varying concentrations of template DNA, dNTPs, Taq DNA polymerase and annealing temperature. The reaction was performed in a volume of 10 μl containing 20 ng template DNA, 0.5mM dNTPs (Chromous Biotech, Bangalore, India), 0.15U Taq DNA polymerase (Chromous Biotech), 0.5μM ISSR primers from University of British Columbia (The Michael Smith Laboratories, University of British Columbia, primer set # 9, Vancouver, BC, Canada) and 1X PCR buffer (10mM Tris HCl pH 8.3, 50mM KCl, 3mM MgCl2 – it is different in Krishna ‘s report) (Merck). A total of 10 primers were used for the present study (Table 2). A control PCR tube containing all components but no genomic DNA was run with each primer to check any contamination. The reactions were carried out in a DNA thermocycler (Eppendorf mastercycler gradient, Germany). The thermocycler was programmed for an initial denaturation step of 95oC for 4 min, followed by 34 cycles at 94oC for 1 min, 45 sec at the specific annealing temperature of each primer (45 to 55âÃâæC) and 72oC for 1 min; and a final extension at 72oC for 8 min and a hold temperature of 4oC at the end. After amplification, the reaction products were subjected to electrophoresis in 1.5% agarose gels in 1x TAE buffer stained with 0.5μg/ml ethidium bromide and photographed under UV light with the help of a Gel documentation system (Syngene). 1Kb molecular ladder was used as molecular marker to know the size of the fragments. All the PCR results were tested for reproducibility by at least three times. Bands that did not show fidelity were eliminated. |
| Data Analysis: Amplified products, which were reproducible and consistent in performance, were scored for band presence (1) or absence (0) and a binary qualitative data matrix was constructed. Pairwise comparisons were calculated from the data matrix and analysis was carried out following UPGMA method using the wards minimum variance algorithm. Genetic diversity was measured by the percentage of polymorphic bands (PPB), calculated by dividing the number of polymorphic bands by the total number of bands surveyed. |
 |
RESULTS AND DISCUSSIONS |
| A total of 80 clear and reproducible bands were amplified with different lengths from twelve populations of G.sylvestre using the 10 selected ISSR primers, of which 68 (85%) were polymorphic and remaining 12 (15%) were monomorphic. The bands were varied between 5-12 with an average of 8 bands per primer. The maximum number of polymorphic bands (10) was obtained using UBC 827 and 834. The primers UBC 866 and 906 showed 100% polymorphism (Table 2). The average polymorphism among polymorphic primers was 83.63%. UPGMA dendrogram based on ward’s method generated one major cluster and one minor cluster with linkage distance of 42 (Fig. 1). |
 |
| The major cluster consisted of two sub clusters. The sub cluster I consisted of sample numbers G1, G3 and G5 whereas the sub cluster II consisted of sample numbers G2, G12, G4, G7, G11 and G8. The two sub clusters of the major cluster joined together at the distance of 28. The minor cluster consisted of G9 and G10. While sample number G6 (Maagadi), which stood apart, joined cluster at 54 linkage distance. The analysis revealed that the samples G1 and G3 were closer while the sample from Maagadi (G6) was most diverse from all other samples. |
| G.sylvestre is a wide spread species throughout Karnataka. Endemic species have the lowest genetic diversity whereas regionally distributed and wind spread species had geographically a history of large and continuous population which was less susceptible to loss of genetic variation due to genetic drift (Contle et al., 1998; Qiu et al., 2005). Since, G.sylvestre is the cross - pollinated species probably it might have higher genetic variation among and within population. By using ISSR primers, we demonstrated that there was considerable genetic diversity within populations of G.sylvestre, 85% of bands were polymorphic in 12 populations. The UPGMA dendrogram clearly depicted spectra of genetic diversity among various accessions. Accession sample collected from Maagadi was most diverse from all other samples. |
| Geographical conditions affect the active constituents of medicinal plants and hence their activate profiles. Estimation of genetic diversity is an important aspect in designing the crop improvement programme as well as management of germplasm and strategies for conservation. Also, it provides information for designing sampling protocols (Bretting and Wilriechner, 1995). The genetic structure of plant populations reflects the interactions of various evolutionary processes including the long-term evolutionary history, such as shifts in distribution, habitat fragmentation, and population isolation, mutation, genetic drift, mating system, gene flow, and selection (Schaal et al., 1998). |
| Several attempts have been made to understand population structure for a number of medicinal plant species to establish commercial level propagation for useful secondary metabolites using molecular markers (Siva and Krishnamurthy, 2005). Among the various molecular methods for detecting genetic diversity, a technique amplifying intersimple sequence repeats (ISSR) is a powerful tool for investigating genetic variation within species (Gupta et al. 2004; Zietkiewicz et al. 1994; Wolfe and Liston 1998). ISSRs are hypervariable as compared to other tools that access genetic variation such as allozymes and RAPDs because of their sensitivity, simplicity and cost-effectiveness (Nayak et al. 2003). Population genetic diversity and its geographic distribution have been assessed in several species of medicinal plants, for instants, Codonopsis lanceolata (Guo et al., 2006), Megacodon stylophorus (Ge et al., 2005), Podophyllum hexandrum (Alam et al., 2008), and Tribulus terrestris (Sarwat et al., 2008) using ISSR molecular marker. |
CONCLUSION |
| In our study, 85% of the total genetic variation was observed among populations of G.sylvestre, which implied the need to conserve more populations to maintain within this species. As an important medicinal plant, it would be wise to save populations of G.sylvestre in different regions in order to limit population declines caused by over exploitation and largescale environmental catastrophes. Further research on this species is required to develop the conservation strategies and improvement programmes. |
References |
|