ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Nimisha Patel1, Yogesh Patel2, and Archana Mankad3
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Soils contain natural reserves of plant nutrients but these reserves are largely in different forms which are used for plant growth. Also, Plant nutrients are not available to plants, because of the extra use of the chemical fertilizers. Chemicals make the nutrients, which remain in soil, in active and so are not used by plants, also make the soil polluted. The using of chemical fertilizer since long time results in the soil being full of chemicals thus damaging the production and full of harmful chemicals to the human body. As an option to all this use of biofertilizers can help us get back our soil health by natural way ultimately the health of organisms.
Keywords |
| Biofertilizer, Soil, Chemical fertilizer. |
INTRODUCTION |
| Biofertilizers are low cost, renewable sources of plant nutrients. These are selected strains of beneficial soil microorganisms cultured in the laboratory and packed in suitable carrier. Biofertilizers are gaining momentum recently due to the increasing emphasis on maintenance of soil health, minimize environmental pollution and cut down on the use of the chemicals in agriculture [1]. Increased crop production largely relies on the type of fertilizers used to supplement essential nutrients for plants. For optimum plant growth, nutrients must be available in sufficient and balanced quantities. From the soil nutrients only a minor portion is released each year through biological activities or chemical processes. Therefore, fertilizers are designed to supplement the nutrients already present in the soil [2].Very often microorganisms are not as efficient in natural surroundings as one would expect them to be and therefore artificially multiplied cultures of efficient selected microorganisms play a vital role in accelerating the microbial processes in soil. Biofertilizers have various benefits. By controlling soil borne diseases, improving the soil health and soil properties, these organisms help not only in saving, but also in effectively utilizing chemical fertilizers and result in higher yield rates [3]. |
II. MATERIAL AND METHOD |
| Preparation of experiments: The soil samples were sieved through a 4.0 mm sieve and filled into earthen pots by 5kg/ pot and mixed with different fertilizers. The ratio taken for soil and fertilizers was 70:30. Then, the selected plant Cuminum cyminum L. was transplanted in three types of the soils. There were randomly saplings in each pot based on the plant size. The experiment was carried out in the Botanical Garden, Department of Botany, University School of Sciences, Gujarat University. The plants were grown in pots with three treatments. In first set chemical fertilizer (urea, ammonia) was added, in another (bacterial, powder) was added and control was maintained without any fertilizer. Watering was done at15 day interval. After 120 days plants were harvested. After harvesting the plants, soil samples were collected from all pots and different parameters (pH, electrical conductivity, organic carbon, nitrogen, phosphorus, potassium, Mn, Zn, Fe and Cu) were determined by different methods, which are as follows: |
ANALYSIS METHODS: |
pH (pouvoir hydrogen) measurement: |
| 20gm of soil was taken in a100ml beaker and 50ml of distilled water is added. The suspension was stirred at regular intervals for 30minutes and pH was recorded by pH meter.(Harrison pH meter) |
Electrical conductivity: |
| 20g of soil was shaken intermittently with 40ml of distilled water in a 150ml conical flask for 1 hr. and allowed to stand. The clear extract after pH determination can be used for electrical conductivity measurement by conductivity meter. (Conductivity meter, CC-01, Contech comp.) |
Organic carbon: (Walkley 1947 and Black 1965)[4] |
| 0.10 to 2.00 g dried soil was taken. Added 10ml of 0.167M K2Cr2O7then added 20 ml of conc. H2SO2 swirled gently to mix. Allowed to stand 30minutes.The flask should be placed on an insulation pad during this time to avoid rapid heat loss.The suspension was diluted with 200ml of distilled water. 10ml of 85% H3PO4 and 0.2g of NaF added. Then 10 drops of ferroin indicator was added. Titrated with 0.5M ferrointo a wine red colour end point. |
Nitrogen: (Kjeldahl, 1883)[6] |
| 1g soil sample into a digestion tube and 0.5g soil rich in organic matter, include two blanks were prepared. Added 2g of catalyst mixture and 7ml of concentrated H2SO4and by swirling. Digest for 3 hrs or until the digest was clear and white or pale yellow. Allowed cooling and continually adding 50ml of distilled water and allow cooling again. Take 10ml of boric acid solution, then added 2 drops of indicator solution and placed the condenser of the steam-distillation unit. Started the distillation process (add 3 strokes of 40% NaOH) wait 0.5 minutes and 2.5 minute steam distillation. When the distillation was completed, Added a stirrer bar and titrate the receiver flask solution from green over colourless to a pink end point with 0.1N H2SO4. Recorded the amount of H2SO4 used. |
Phosphorus: (Apha, 1998)[7] |
| 35ml of aliquot was taken in 50ml volumetric flask then added 10ml of vanadate – molybdate reagent and diluted to the mark with distilled water. Blank was prepared in distilled water. After 10min absorbance of sample was measured at 400nm wavelength and determined by comparison of the reading with a standard curve. |
Potassium as k+: (Apha, 1998) [7] |
| A blank and potassium calibration standards were prepared in the range of 0.1 to 1.0 mg/l. Emission intensity was determined at 766.5 nm by flame photometer. Calibration standards and aliquot were aspirated enough time to secure a reliable average reading for each. Calibration curve was constructed from the potassium standards. Potassium concentration of the aliquot was determined from the calibration curve. |
Mn, Zn, Fe and Cu: (Jackson, 1965)[8] |
| 0.5gm soil sample was taken and mixed with acidic solution of conc. HNO3 and conc. HCl4 (Jackson, 1965) [8] and was kept overnight. Next day flask heated on hot plate and toward the end of digestion the flask was brought near dryness. The solution was made to 20ml with double distilled water then it filtered through whatman no.44 filter paper and makes 100ml final volume with double distilled water. The blank and the soil sample were analyzed in atomic absorption spectrophotometer and the concentration of heavy metal namely Mn, Zn, Fe and Cu were analyzed.s |
III. RESULT AND DISCUSSIONS |
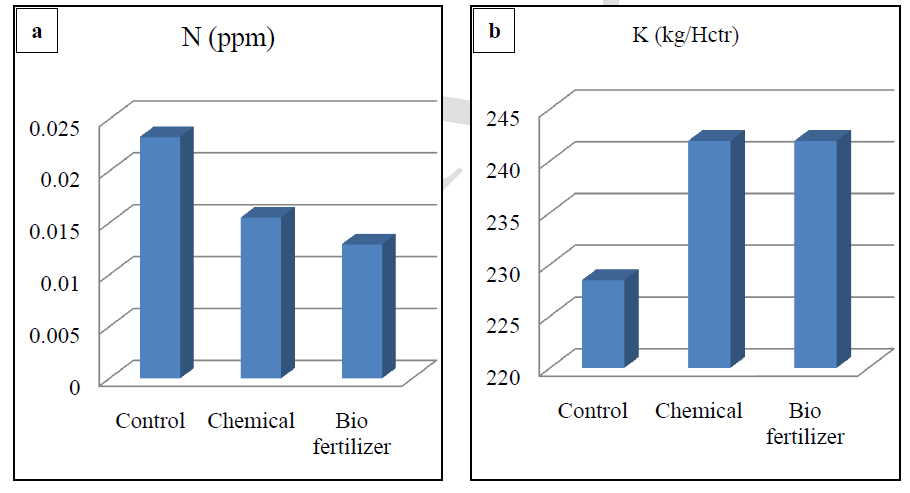
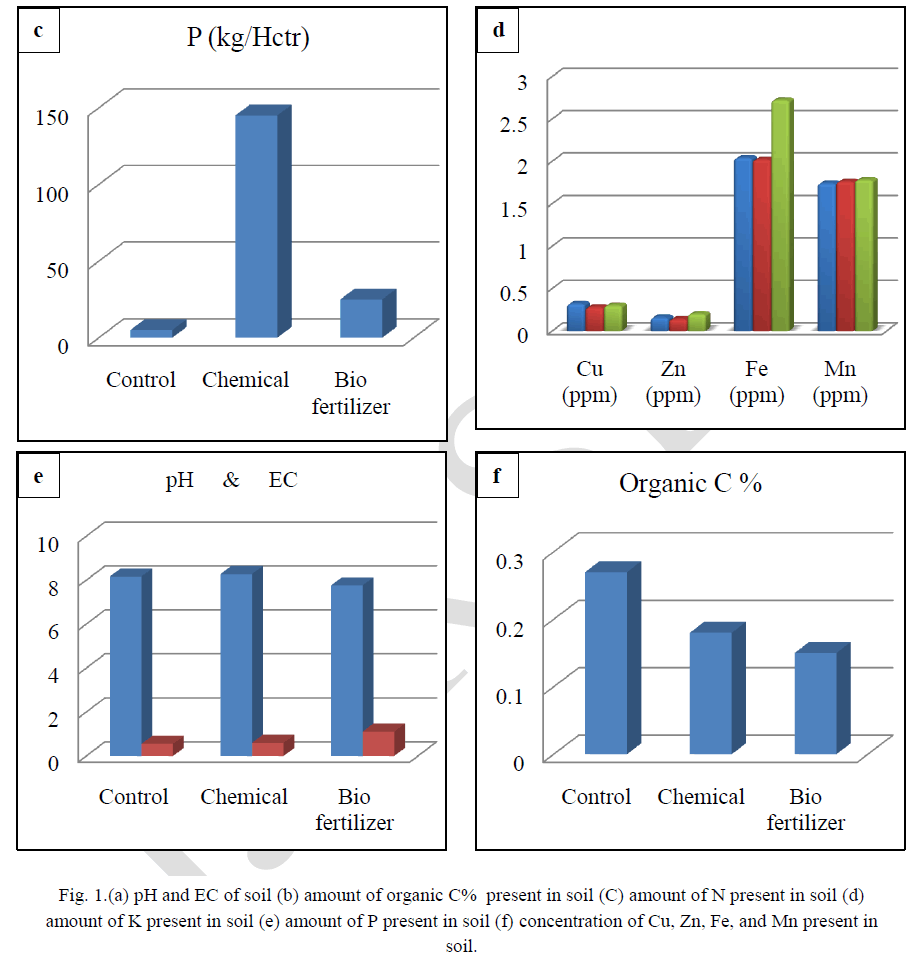
| Soil testing is referred to as chemical analysis of soils and is well recognized as a scientific means per quick characterization of the fertility status of the soil and predicting the nutrient requirement of the crop. Results show that in biofertilizer treatment results in relatively low pH, which is optimum for solubilisation of bound elements. Soil becomes alkaline after the treatment of chemical fertilizers. There is reduction of solubility of many nutrients (Figure-a) and the soil treated with biofertilizer had high EC which is optimum for better plant growth (Figure-a).Results shows that highest value of organic carbon in control followed by chemical fertilizer treated soil and least value in biofertilizer treated soils. (Figure-b).Highest concentration of the nitrogen was found in control, followed by treated soil with chemical fertilizer and lowest in treated soil with biofertilizer. The use of Chemical fertilizer has decreased the in recent years, although it is a very good source of nitrogen. Application of biofertilizers decreases the nitrogen value in soil (Figure-c). Chemical and biofertilizer treated soil results in optimum value of potassium which is less in control condition (Figure-d).It was recorded that the chemical fertilizer treated soil shows highest concentration of phosphorus and biofertilizers treated soil shows optimum concentration of phosphorus. On the other hand less concentration of phosphorous was recorded in control condition. Excess availability of phosphorus in soils is often limited by fixation reactions, which convert the ion into various insoluble forms and optimum availability of Phosphorus compounds act as energy currency within the plants and involve in wide range of plant processes from permitting cell division to developing good root system. Dutta et al,(2003)[9] reported that NPK played significant role in sustaining soil fertility and productivity of crop. Also a higher positive effect on microbial biomass and hence soil health. Similar result were found by Chand et al, (2006)[10] and Bokhtiar and Sakurai, (2005)[11].Figure-f shows that application of biofertilizer increases the level of micronutrients (Cu, Zn, Fe and Mn) in soil and makes the soil healthy as compared to the chemical fertilizer. |
 |
 |
IV. CONCLUSION |
| This information is important for determining whether the soils could supply adequate nutrients for optimum crop production or not. Soil testing helps in understanding the inherent fertility status of the soil. Biofertilizer plays an important role in supplementing nutrients to the plants and restoring the soil fertility, regenerates soil productivity leading to the sustainable farming. |
References |
|