ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Sunita J. Varjani1 and Vivek N. Upasani2*
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Globally environmental scientists have shown increased interest in promoting biological methods referred to as “Green technology” in the clean-up technologies for hydrocarbon-polluted sites. These methods are less expensive and do not introduce additional chemicals/pollutants into the environment. Compared to physico-chemical methods, bioremediation / biodegradation offer a feasible alternative for this purpose to the petroleum industry. One reason is that the majority of the molecules in the crude oil and refined products are biodegradable. We have reported the isolation of Hydrocarbon Utilizing Bacteria (HUB) from crude oil contaminated soil and Untreated Effluent water (UEW) samples collected samples from selected ONGC sites in Gujarat state. Screening for hydrocarbon utilizing capacity of these bacteria was carried out using Bushnell-Hass (BH) medium with crude oil (K#X) and redox indicator 2, 6 - dichlorophenol indophenol (DCPIP). Maximum utilization of hydrocarbons from crude oil was indicated by the total discoloration of DCPIP. In total five consortia (Hydrocarbon Utilizing Bacterial Consortium: HUBC) named HUBC-S, HUBC-W, HUBC, HUBC-Ank, and HUBC-AH were prepared to screen for the most efficient consortium for hydrocarbon degradation. Our results indicated that the consortium HUBC consisting of six isolates showed best results. Total discolouration of DCPIP by “HUBC” and its individual isolate was about 53 h and 120 h, respectively. The results of this study are presented here.
Keywords |
| BH medium, Biodegradation, Consortia, Crude oil, HUBC, UEW, DCPIP |
INTRODUCTION |
| Contamination of soil, water and sediment samples with petroleum hydrocarbons has resulted in environmental and health hazards. Clean up/remediation of such pollutants requires the development and implementation of innovative technology [1]. Bioremediation is proposed as an alternative to various physico-chemical treatments at many hydrocarbon contaminated sites [2]. Biodegradation of petroleum hydrocarbons is a complex process that depends on the type and amount of hydrocarbons present [3]. Diverse microorganisms (strains of bacteria, fungi, yeast, algae etc.) have been reported to have the capability to degrade the hydrocarbon pollutants. Significant role of native flora in biodegradation / bioremediation is also reported in literature. The most rapid and complete degradation of the majority of organic pollutants is brought about under aerobic conditions [4-9]. Limited range of petroleum compounds is metabolized by individual microorganisms; mixed populations with variety of enzymatic capacities are required to bring the quality and quantity of hydrocarbon utilization /degradation. Microbial populations that consist of strains that belong to various genera have been detected in hydrocarbon-contaminated soil, water or sediment samples. This strongly suggests that each strain or genus has specific role in the degradation processes [1-3, 10-12] Screening of individual microbe / consortium for utilization/degradation of pollutants is an important task to develop the bioremediation processes [13]. 2, 6-dichlorophenol indophenol (DCPIP) based colorimetric assay can be used to study the utilization / degradation of hydrocarbons by microorganisms. In oxidized state the indicator is blue and its reduced state is colourless. The colour change is due to structural change in the molecule, in which the double bond between nitrogen and carbon passes to a single bond. The colour change is directly proportional to utilization / degradation of hydrocarbon compound (s) [7, 9, 13, 14]. Present study represents investigation of utilization of hydrocarbon contaminants / pollutants by indigenous bacterial isolates individually and their consortium. Five bacterial consortia were prepared which were denoted as HUBC-S, HUBC-W, HUBC, HUBC-Ank, and HUBC-AH. The strains were selected based on the criteria that they were able to perform discoloration of DCPIP (utilization of hydrocarbon component (s) of the crude oil in a relatively short time). |
MATERIALS AND METHODS |
| Chemicals and Carbon substrate: DCPIP was purchased from Sisco Research Laboratories (SRL, Mumbai, India). Bushnell- Hass (BH) and Nutrient agar media were procured from HiMedia (Mumbai, India). Other reagents used in this study were of analytical grade. |
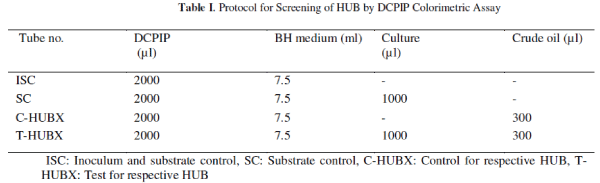
| Screening of HUB: Crude oil contaminated soil, Untreated Effluent water (UEW) were collected with the technical assistance of ONGC authorities from various oil fields of ONGC sites located in Ahmedabad and Ankleshwar Asset of Gujarat. In total 69 HUB were obtained by enrichment culture technique as described elsewhere [9]. The utilization of respective crude oil by all 69 isolates was evaluated using the technique based on the redox indicator DCPIP. Modified Hanson et al. method was used to screen potential HUB [9, 14]. Activated bacterial cultures were inoculated into tubes along with 0.5% v/v DCPIP indicator, and 3% v/v respective crude oil for spectrophotometric analysis. All the tubes were incubated at room temperature for six days. Appropriate controls and substrate / crude oil assays were included as mentioned in Table 1. |
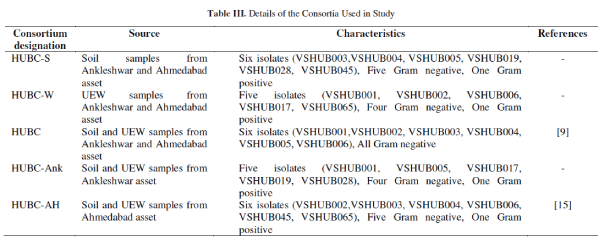
| Microbial consortia preparation and Analysis: Based on the time for discolouration of DCPIP one isolate was selected from each sample viz. soil or UEW sample(s). Isolates which decolorized DCPIP in the relatively shortest time (about 120-125h) were chosen for preparing a consortium. Consortium preparation is described elsewhere [15]. Five such consortia were prepared using the most efficient isolates and designated as HUBC-S, HUBC-W, HUBC, HUBC-Ank, and HUBC-AH. For selection of the most effective consortium crude oil from well K#X was used as sole carbon source, rest of the contents of all assay tubes remains the same as shown in Table I. All individual isolates and consortia were studied for decolourization of DCPIP [13]. Discoloration of DCPIP is directly proportional to the hydrocarbons utilization of hydrocarbons by the individual isolate/consortium. The absorbance means optical density of all the assays was measured spectrophotometrically by Hach® DR2500 spectrophotometer at 600nm. Data was collected at regular time interval of 24h till six days. |
RESULTS AND DISCUSSIONS |
| Biodegradation studies to check the ability of mixed microbial populations for the utilization of hydrocarbon(s) in crude oil as the sole carbon source have been reported by many authors [1, 3, 7, 11, 15, 16]. Bacteria are the primary degraders and most active agents in petroleum degradation. Several bacteria are even known to grow exclusively on hydrocarbons [17]. Interest in the potential of biodegradation and bioremediation technology was increased after success of bioremediation efforts in the cleanup of the oil spill due to Exxon Valdez oil tanker disaster in 1989 at Prince William Sound, Alaska [16]. The bioremediation of soils and sites contaminated by crude oil is often limited due to the scarcity of indigenous specialized microbes with the complementary substrate specificity required for degrading the different hydrocarbons present at the contaminated site [12]. Many times due to accumulation of recalcitrant compounds and low availability of hydrocarbons complete hydrocarbon degradation is not possible in field conditions [3]. Lubricant oil compounds such as cyclic alkanes are not biodegraded by only one microorganism. Hence, a consortium having a group of potential oil metabolizing microorganisms is required for this purpose [13]. The degradation of petroleum hydrocarbons can be mediated by specific enzyme system, the initial attack on xenobiotics is done by oxygenases. Mainly two mechanisms are reported to show involvement of microbes for biodegradation of hydrocarbon pollutants, one is direct (attachment of microbial cells to the substrates) and the other is indirect (through production of micro nutrients or biosurfactants). The former mechanism is still unknown, but the later has been well studied [16]. Production of biosurfactants (surface-active agents) for the solubilization of aliphatic or aromatic hydrocarbons by Pseudomonas sp. is reported by several authors [8,18,19]. |
| Samples used in this study were collected from ONGC sites located at Ankleshwar and Ahmedabad asset in Gujarat state. Crude oil contaminated subsurface soil samples were collected near oil wells, Gas Gathering Stations (GGS) and Central Tank Farm (CTF); UEW (Untreated Effluent Water) samples were collected from GGS and CTF, whereas Crude oil samples were collected from the mouth / surface opening of oil wells, GGS and CTF. Sixty nine (69) HUB isolates were obtained from six crude oil contaminated soil and five UEW samples of Ankleshwar and Ahmedabad assets in Gujarat state. Out of sixty nine (69) HUB isolates, forty (40) were obtained from Ahmedabad asset. Out of forty (40) HUB isolates, twenty three (23) were obtained from soil samples whereas seventeen (17) were obtained from Untreated Effluent water (UEW) samples. Twenty nine (29) isolates were obtained from Ankleshwar asset, out of which nineteen (19) were obtained from soil samples whereas ten (10) were obtained from UEW samples. Out of sixty nine (69) HUB isolates, forty two (42) isolates were obtained from soil samples, whereas twenty seven (27) were obtained from UEW samples. |
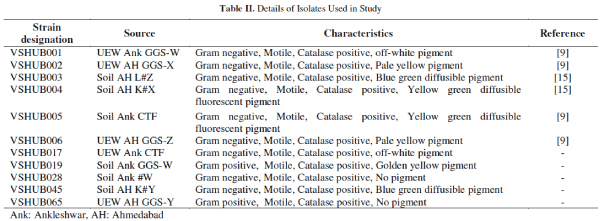
| Screening for potential hydrocarbon utilizing bacteria by DCPIP assay has been reported [4, 7, 9, 14, 15, 20]. DCPIP is an enzyme-catalyzed oxidation electron acceptor and pH dependant redox indicator; it is blue when oxidized and colourless when reduced. Its loss of colour is monitored spectrophotometrically at a wavelength of 600nm [13]. As microorganisms utilize hydrocarbon(s) present in crude oil, DCPIP is reduced and due to this as time passes after inoculation DCPIP become colourless. All the sixty nine (69) isolates were screened for their efficiency to utilize respective crude oil (as a sole carbon source) using DCPIP indicator. Colour change of DCPIP was observed periodically at interval of 24h till six days visually and optical density was measured. Based on the time for discolouration of DCPIP one isolate was selected from each sample viz. soil or UEW sample. All isolates were not able to decolorize DCPIP completely in six days. DCPIP discoloration time for individual isolates was varied from 120 - 144h (data not shown). Isolates which decolorized DCPIP in the shortest time (about 120-125h) were chosen for preparing the consortia. Details of the selected isolates and prepared consortia are represented in Table II and Table III, respectively. Selected individual isolates and their consortia were again studied for discoloration of DCPIP using crude oil from well K#X. The crude oil samples contained moisture content, saturates, aromatics, resins and asphaltenes in the range 0 - 24%. 77.2 - 80.8%, 12.4 - 17.8%, 3.2 - 8.8% and 0.8 - 4.3%, respectively, as reported elsewhere [9]. |
| There are various reports available on metabolic versatility of mixed cultures that has demonstrated superiority of mixed cultures to pure cultures [7, 21, 22]. Moneke and Nwangwu (2011) reported isolation of hydrocarbon utilizing Pseudomonas sp, Bacillus sp. and Klebsiella sp. They performed biodegradation experiment for single and mixed culture using kerosene, engine oil and automotive oil as sole source. They concluded that use of native microorganism(s) for biodegradation is beneficial and profitable [22]. Malik and Ahmed (2012) studied mixed consortium of 15 bacteria, isolated from an oil contaminated site of Pakistan and reported 94.64% and 93.75% removal of aliphatic and aromatics, respectively; of the used crude oil after 24 days of incubation [23]. Necessity of consortium with broad enzymatic capacities for degradation of complex hydrocarbon pollutants such as crude oil or diesel fuel was also reported. Fifty seven (57) bacteria degrading crude oil from the hydrocarbon polluted sites of gasoline and diesel spilled gas stations of Coimbatore, India have been isolated. Screening lead to selection of one Bacillus sp., one Corynebacterium sp. and two Pseudomonas sp. The bacterial consortium showed better degradation as compared to that of the individual strains. Using 1% crude oil at 30°C after incubation of 25 days mixed bacterial consortium degraded 77% of the crude oil, whereas degradation by individual bacterial strain ranged from 69%- 41%. The variety of microorganisms and their degradative enzymes help in the degradation of aromatic hydrocarbons by a microbial consortium [11]. Bao et al., (2012) reported a consortium of Ochrobactrum sp. and three Brevibacillus parabrevis sp. can degrade 79% of crude oil after 14 days of incubation under standard experimental condition (s) [24]. Varjani and Upasani (2012) reported utilization of crude oil by consortium obtained from crude oil contaminated soil samples of selected ONGC sites of Ahmedabad asset. Under standard experimental conditions the complete discolouration of DCPIP was observed in 72 h of incubation. Two isolates from these six isolates constituting the consortium were identified as Pseudomonas sp [15]. |
| There are several reports available for wide distribution of petroleum-degrading microorganisms in soil, water and sediments but they may not be present in sufficient numbers to achieve contaminant remediation [17]. Contaminant removal can be achieved by inoculation of the polluted area with highly effective hydrocarbon-degrading microbial strains to augment / stimulate the existing ones [2]. Isolation of Acinetobacter sp, Bacillus sp., Micrococcus sp., Pseudomonas sp. Streptomyces sp., and fungi Aspergillus sp, Trichoderma sp, Penicillin sp. from diesel oil polluted soil sample from Pravara river bed around Sangamner city, Western India and their ability of a consortium of all isolates for biodegradation of crude oil using BH medium and DCPIP has been reported [1]. |
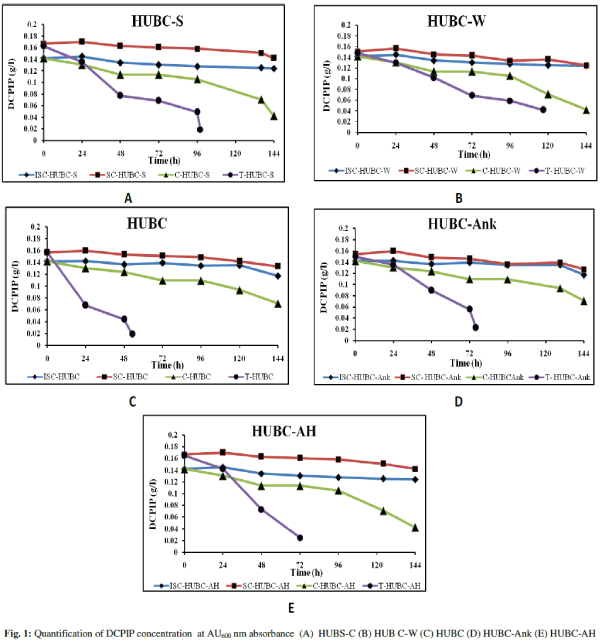
| “ISC-HUBX” and “SC-HUBX” did not show discoloration, whereas the substrate assays (“C-HUBX and “T-HUBX”) (from this point X will be referred as C-S, C-W, C, C-Ank, and C-AH for respective consortium) showed remarkable discolouration (in terms of oil utilization). It was interesting to note that the “T-HUBX” assay completely decolourized DCPIP for all the consortia tested. The biodegradation time observed for the consortia HUBC-S, HUBC-W, HUBC, HUBC-Ank, and HUBC-AH was 98 h, 117 h, 53 h, 76 h and 72 h, respectively (Fig. 1A to 1E). Finally “HUBC” and its six isolates were further studied for decolourization of DCPIP spectrophotometrically by measuring absorbance at 600 nm, periodically [13]. The time required for total discoloration of DCPIP for “HUBC” was less than half required by pure cultures of the isolates used for preparation of the consortium (Fig. 2A to 2D). DCPIP concentration below 0.030 g/l was considered as complete discolouration. |
| Ghazali et al., (2004) reported comparative study of two consortia prepared from the isolates obtained from Center for Research in Enzymes and Microbiology (CREAM), Malaysia. Consortium No. 2 consisted of three Bacillus sp., two P. aeruginosa strains, and one Micrococcus sp. whilst consortium No. 1 consisted of one Bacillus and two Pseudomonas strains. As compared to consortium No. 1, consortium No. 2 proved more efficient for the cleanup of the medium- and long-chain alkanes in the dieselcontaminated soil of Selangor, Malaysia. They proposed the important role of Bacillus strains for more extensive biodegradation. In their study they reported 57% removal of tetradecane by consortium No. 2 after 60 days of study. The use of individual pure cultures and mixed cultures of hydrocarbon degrading microorganisms has been tested with interesting results on hydrocarbons contaminated soils [3]. |
| A small number of experimental trials were performed for the application to off-shore environments. Priority must be given for the development of proper method to establish the applicability of such bioremediation agents. In this paper, we have compared the potential of five bacterial consortia obtained from the oil-polluted soil and UEW samples of selected sites of ONGC from Gujarat. The results showed that among all five investigated consortia, “HUBC” decolorized DCPIP in the shortest time (53 hours of incubation), which is highly appreciable. From the results obtained here it can be said that mineralization of hydrocarbon pollutants require co-presence of different bacterial species, having different metabolic pathways for degradation of specific pollutant present in crude oil. |
 |
 |
 |
 |
 |
CONCLUSION |
| The consortia prepared here showed faster utilization of DCPIP than the individual bacterial strain used to prepare it. This could be due to synergistic action of the isolates or due to the different metabolic pathways employed by the individual bacterial species. Among the five consortia studied “HUBC” consisting six strains isolated from crude oil contaminated soil and UEW samples gave best results. Under the experimental conditions time required for utilization of crude oil components as sole source of carbon by “HUBC” was less than half of that required by the individual isolates. Further optimization of this consortium will lead to an effective and eco-friendly technology for the degradation of hydrocarbons from crude oil. |
ACKNOWLEDGEMENT |
| We are thankful to the authorities at Ahmedabad Education Society (AES), M. G. Science Institute, Indian Institute of Advanced Research (IIAR) for providing laboratory facilities. We would like to thank the officers Institute of Reservoir Studies (IRS), ONGC, Ahmedabad, Gujarat for their invaluable suggestions. The suggestions provided by Dr. M.C. Sharma, Biotechnology Department, Kadi Sarva Vishwavidyalaya, Gandhinagar are highly acknowledged. |
References |
|