E- ISSN: 2320 - 3528
P- ISSN: 2347 - 2286
E- ISSN: 2320 - 3528
P- ISSN: 2347 - 2286
Department of Biochemistry, Kebbi State University of Science and Technology, PMB - 1144, Aliero, Nigeria.
Received date: 16/09/2012 Accepted date: 11/11/2012
Visit for more related articles at Research & Reviews: Journal of Microbiology and Biotechnology
Sodium Dodecyl Sulphate is a widely used detergent in the areas of Biotechnology, Biochemical analysis, surfactant chemistry and polymer technology. This review focuses on literature reports on the current trends and future application of SDS in the aforementioned areas.
SDS, Biotechnology, surfactant, Biochemistry, polymer.
The most common sulphate surfactant is sodium dodecyl sulphate (abbreviated as SDS or NaDS and sometimes referred to as sodium lauryl sulphate abbreviated as SLS), which is extensively used both for fundamental studies as well as in many industrial applications [1]. The salt consists of an anionic organosulfate consisting of a 12-carbon tail attached to a sulfate group, giving the material the amphiphilic properties required of a detergent. SDS is a highly effective surfactant and is used in any task requiring the removal of oily stains and residues [2]. SDS is a synthetic detergent that differs from ordinary soaps that are produced from the hydrolysis of fats in a chemical reaction called saponification [3], or base promoted hydrolysis of fats and oils [4].
It is used in industrial products such as car wash soap, engine degreasers and floor cleaners. SLS is an ingredient in a wide range of personal care products such as soaps, shampoos and toothpastes. SLS is added to soaps, bubble baths and toothpastes for its thickening effect and its ability to create lather and as creams and lotions. In this function, surfactants wet body surfaces, emulsify or solubilize oils, and suspend soil. It is used in so many products because it is a cheap, highly effective cleansing and foaming agent.
Production of SDS
SDS can be prepared by reacting dodecyl alcohol (dodecanol) with sulfuric acid [5].
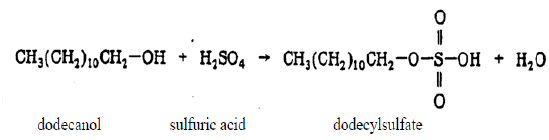
The resulting dodecyl sulfate is converted to the sodium salt by a reaction with sodium hydroxide.
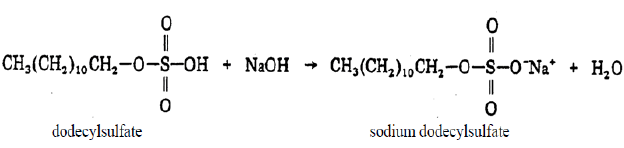
Application of SDS Detergent In Biochemistry and Biotechnology.
SDS detergent is used in Biotechnology for numerous applications. It is commonest detergent frequently used in Recombinant DNA technology. It is regarded as a membrane solubilizer; it solubilizes the membrane so that all the components of DNA will be in solution. A sulfuric acid ester group in the sulfate ester surfactant acts as the solubilizing group.
The most commonly used method of DNA isolation utilizes lysis of cells by detergent SDS. SDS disrupts cells membrane releases the DNA into the solution. In the isolation and analysis of chromosomal DNA, SDS is added to denature proteins and solubilize lipids in membranes leading to lysis. In sodium dedocyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) a technique use in Biotechnology to separate proteins according to their mobility, the solutions of protein to be analyzed is first mixed with SDS. SDS function to bind to and unfolds the protein, given rise to uniform negative charge along the length of the polypeptide.SDS masks the proteins natural net charges and gives all proteins the same charge –to mass ratio, the proteins separates based on molecular weight instead of charge.
Most commonly, the strongly anionic detergent sodium dodecyl sulfate (SDS) is used in combination with a reducing agent and heat to dissociate the proteins before they are loaded on the gel. The denatured polypeptides bind 50S and become negatively charged. Because the amount of SDS bound is almost always proportional to the molecular weight of the polypeptide, and is independent of its sequence, 50S-polypeptide complexes migrate through polyacrylamide gels in accordance with the size of the polypeptide. At saturation, approximately 1.4 g of detergent is bound per gram of polypeptide. By using markers of known molecular weight, it is therefore possible to estimate the molecular weight of the polypeptide chains [6].
For highly expressed proteins, solubilization yield can be estimated by SDS-PAGE with Coomassie Blue staining [7]. Modern applications of the technique of SDS-PAGE facilitate the study of such proteins in a rapid and efficient manner. The porosity of the gel formed as a result of the copolymerization of acrylamide with the cross-linkerbisacrylamide acts as a molecular sieve in which macromolecules of various size and charge can be separated [8].
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) is the most commonly used procedure for checking the purity of proteins. In electrophoresis, molecules move in an electrical field. Normally, the speed of their movement depends on three factors—their size, their shape, and their electrical charge.
In SDS-PAGE, the protein mixture is treated in such a way that only the molecules’ mass affects their movement. This is achieved by adding sodium dodecyl sulfate (C12H25- OSO3Na), the sulfuric acid ester of lauryl alcohol (dodecyl alcohol). SDS is a detergent with strongly amphipathic properties.. It separates oligomeric proteins into their subunits and denatures them. SDS molecules bind to the unfolded peptide chains (ca 0.4 g SDS / g protein) and give them a strongly negative charge. To achieve complete denaturation, thiols are also added in order to cleave the disulfide bonds [9]. Determination of the the number and size of the insecticidal crystal proteins (ICPs) synthesized by four isolates of entomopathogenic spore-forming bacteria conducted using Sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) was reported [10].
One of the common active ingredients in laundry detergent is SDS, and is one of the synthetic detergents most commonly used in Biochemistry. SDS and other detergents like Cetyltrimethylammonium bromide(cationic) and Triton X-100(nonionic) are used,example of a practical application of these detergents in biochemistry is in investigating the effect of a range of concentration of the detergents on the light scattering properties of the lysosomal suspension [11]. Among bio-chemical techniques, sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDSPAGE) is most widely used due to its validity and simplicity for describing genetic structures of group of plants. The use of , SDS-PAGE to confirm sunflower hybrids was reported [12].
Sodium Dodecyl Sulphate in Surfactant Chemistry
Surface-active compounds commonly used in industries are chemically synthesized. However, biosurfactants have been paid increasing attention to replace the synthetic surfactants owing to their advantages such as biodegradability and low toxicity. Nowadays, the use of biosurfactant has been limited due to the high production cost [13].
The science and the technology of surfactants have possibly suffered a double blow from the functional divergence of academic and applied research. Academic interest in surfactants, while increasing, has generally concentrated on highly purified, homogeneous materials [quite often limited to a few materials such as sodium dodecylsulfate (SDS), SDS, have found application in a number of areas related to emulsion polymerization and, of course, represent probably the most intensively characterized family of surfactants known[14]. The results of most studies with SDS and similar surfactants indicate that the more hydrophobic the polymer, the greater is the interaction with anionic surfactants [14]. Dodecane sulfuric acid ester, a strongly acidic compound with good water Solubility when neutralized with alkali, certain alkaline earth metals, or organic amines, the material becomes highly soluble in water and an excellent surfactant. It is, in fact, probably the most extensively studied and best understood surfactant known to science—sodium dodecylsulfate (SDS):
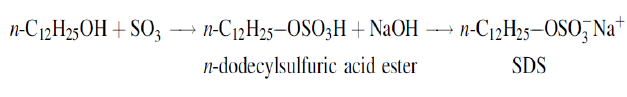
Poly (maleic acid/octyl vinyl ether) (PMAOVE) was found to interact with sodium dodecyl sulfate (SDS) to yield enhanced viscosity and reduced surface tension. This is attributed to the coexistence of the mixed micelles of PMAOVE and SDS.
This behavior of PMAOVE, as shown in Figure 1A and B, is noteworthy for its potential as a thickener in hair-styling gels.
Fig 1 (A) Surface tension of aqueous solutions of sodium dodecyl sulfate in the absence (b) and presence (a) of 0.1% (wt/wt) PMAOVE as a function of SDS concentration. S.D. = ̱̉ 2%. (B) Relative viscosity of aqueous solutions of sodium dodecyl sulfate in the absence (b) and presence (a) of 0.1 % (wt/wt) PMAOVE as a function of SDS concentration. S.D. = ̱̉ 2%. [15]
Solubilization with SDS in Polymer–Surfactant Interactions
Studies have shown that the amounts of a hydrocarbon such as isooctane taken up by complexes of bovine serum albumin (BSA) and sodium dodecylsulfate (SDS) depended linearly on the number of surfactant molecules bound to the protein molecule. They also showed that a minimum SDS cluster size of about 30 molecules bound to the BSA was required before any solubilization occurred. The solubilization isotherms for BSA/SDS also differed from those of SDS micelles alone. Those results indicate that, for the same isooctane activity, the BSA/SDS complexes had a greater solubilizing power than did the micelles. In a similar way, it has been found that the addition of a polymer to a surfactant solution increased its solubilizing power, although no clear-cut correlations were established between the chemical structure of the polymer and its effect on solubilization. In general, larger effects are observed for aromatic than aliphatic materials. The present state of knowledge in this area is not sufficient to allow quantitative predictions to be made about the solubilizing properties of surfactant–polymer complexes based solely on chemical composition, although it is known that their effectiveness depends on the nature of the polymeric component and the polymer: surfactant ratio [14].
In the study of surfactant–polymer interactions in emulsion polymerization for example, the ability of surfactants to form complexes with polymer chains may also affect the ultimate properties and stability of the resulting polymer, especially when the macromolecule exhibits some affinity for or reactivity with water. Perhaps the best documented case of the effect of surfactant on latex stability is that of polyvinyl acetate (PVAc). The stability of PVAc latexes has been found to vary significantly depending on the surfactant employed in its preparation. It has also been found that PVAc could be dissolved in concentrated aqueous solutions of SDS and that it did not precipitate on dilution. The results suggest that, in this case at least, solubilization did not occur in the micelle, but that extensive adsorption of surfactant onto the polymer chain was required. They also indicate that a strong, stable PVAc– SDS complex is formed that produces a water-soluble structure that is essentially irreversible, unlike normal micelle formation. Cationic and nonionic surfactants had little or no solubilizing effect under identical conditions, indicating the specific nature of many, if not most, polymer–surfactant interactions. PVAc is, of course, the precursor for the preparation of polyvinyl alcohol (PVA). The fact that surfactants such as SDS can promote the solubilization of polyvinyl acetate has been used to explain the observed increase in the rate of hydrolysis of PVAc polymers prepared with that surfactant relative to materials prepared with a less strongly interacting polyethylene oxide( POE) –sulfate surfactant. The hypothesis is that the SDS adsorbs onto and solubilizes the polymer surface, leading to a partial swelling of the particles, giving greater exposure to the hydrolytic environment, and loss of surfactant available for particle stabilization. As the surface of the PVAc particle is ‘‘eaten away’’ by the solubilization–hydrolysis process, the interior material becomes more exposed and subject to hydrolysis. The non solubilizing surfactants, on the other hand, would not facilitate the hydrolysis process, reducing the rate of PVAc exposure to reactants in the aqueous phase. They would also remain adsorbed at the particle surface and continue to perform their function as stabilizers [15].
Considering the potential of SDS, concise and condense information was provided in order to add to scientific data base a shift from home and laundry usage of Sodium Dodecyl Sulphate to Biotechnological, Surface Active Chemistry and Polymer Science applications.