ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Sharma, M.1, Gaur, R. K.1, Bhattacharjee, R. R.2
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Biofouling, in general, refers to the undesirable accumulation of microorganism, algae, plants and animals on wetted structures. The same can cause bacterial infections in medical appliances. Current antifouling agents used are costly, toxic, unstable and mainly used in non medicinal applications. Here we have coated surface of glass and some medical appliances using a simple chemical surface modification technique based on dip-coating and studied their antibacterial and antifouling properties. This work will lead to a simplified and scalable technique towards designing benign and antifouling surfaces for any medical applications.
Keywords |
| Biofouling, dip coating, antibacterial and antifouling. |
I. INTRODUCTION |
| The attachment of microorganisms (bacteria, algae, and fungi) and macro organisms (barnacles, sponges, fishes, seaweed, etc.) on wetted surfaces is commonly referred to as biological fouling. It starts with biofilm formation which is a major problem in marine industry and medical field. Biofouling greatly reduces the efficiency of materials and equipment: it physically damages equipment (abrasion/brittleness/increased load). It is responsible for plaque formation that lead to dental caries1, urinary tract infection2, cystic fibrosis3, Staphylococcus osteomyelitis4, middle ear infection, infectious kidney stones and chronic prostatitis5. Preventing biofouling also helps in keeping surgical instruments free of infectious microorganisms. This can lead to healthy medical services. Even bioimplants coated with antifouling coatings can help reduce the risk of cross infections. There is a real need for the continuous development of new non-toxic antifouling formulations. An ideal formulation would have the following properties: permit at least five years biofouling life cycle control, durable and resistant to damage, repairable, low maintenance, easy to apply, hydraulically smooth, compatible with existing anticorrosion coating, cost effective, non-toxic to non-target species, and, effective at port and sea6.The process commonly used to make these medical devices benign is autoclave. In this work we have shown that autoclave alone is not enough to make surfaces bacteria free especially in Indian conditions. Our method is a bioinspired method where we have used ammonium terminated molecules as coating agents and studied their antibacterial effect. The origin of this work lies in designing surfaces with controlled hydrophilicity/hydrophobicity and looking into its effect on biofouling. |
II. EXPERIMENTAL |
A. Modification of glass slide: |
| All reagents were obtained from CDH and used as obtained. Water used was triple distilled water (CDH). The cleaning and treatment of glass slides has been already reported by us7. The slides were cleaned with liquid soap then washed and sonicated with water for 20 min followed by sonication with 1:1 Acetone: Methanol solution. |
| Then sonication with Piranha solution (H2SO4:H2O2: 7:3) for 20 min. Then water jet wash followed by sonication in water for 20 min and dried. Eventually dried in oven and then kept in 1:1 solution of ammonia and hydrogen peroxide for 24 h (Medical devices were only treated with ammonia/peroxide solution). Finally washed with water and dried in oven. These were used as starting surfaces to be dipped into solution of CTAB (0.5mM), CTAB (1.5mM), PDDAC (0.1mM in 1M NaCl), EDTA (2mM). ‘Plasma’ coating technique was also used to modify surface of glass slides and then dipped into CTAB solutions with the above specifications. Contact angle measurements were performed by putting a drop of water on modified glass surface and observed the wetting properties of the water drop on glass surface using a Goniometer. |
B. Antibacterial test on Modified glass Slides: |
| Bacterial colony count assays were conducted on modified glass surfaces for antimicrobial activity. Aqueous suspensions of E. coli, Pseudomonas spp. and Staphylococcus spp. with concentrations of ~ 106cells/ml were sprayed on the test surfaces, dried in air for 2 min and placed in sterile petri dishes. They were covered by molten nutrient agar, allowed to solidify and then incubated at 37º C overnight. The number of bacterial colonies was counted. |
C. Antibacterial test on medical devices: |
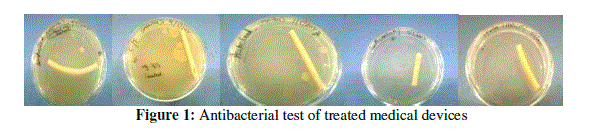
| Bacterial colony count assays were conducted on chemically treated, autoclaved and sterile packed medical devices for antimicrobial activity. Test sample were leaved into open air for 24 hr placed in sterile petri dishes. They were covered by molten nutrient agar, allowed to solidify and then incubated at 37º C for 24-48hr. The number of bacterial colonies was counted. |
III. RESULTS AND DISCUSSION |
A. Antibacterial tests on modified glass surfaces: |
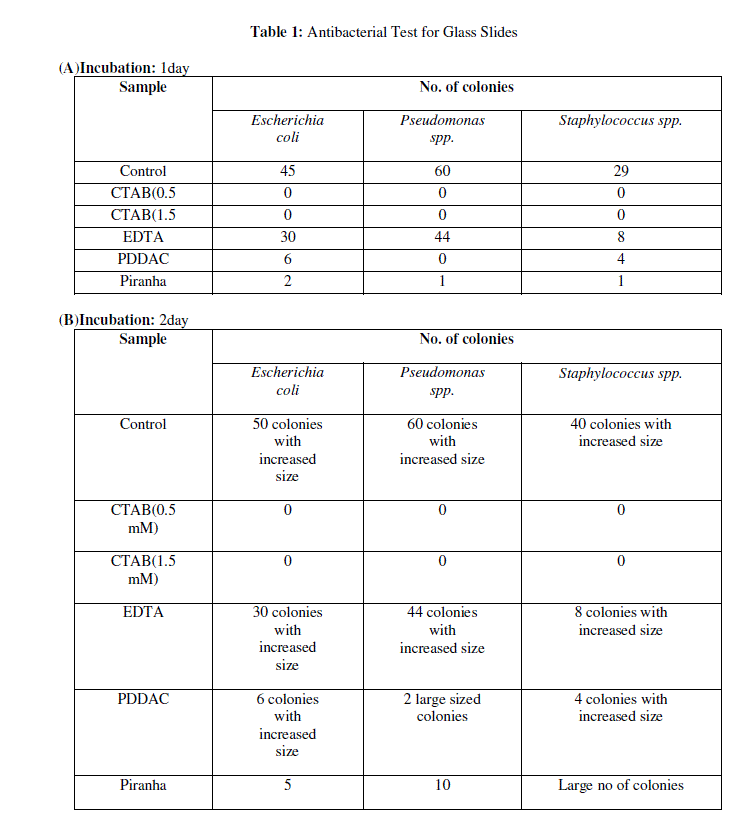
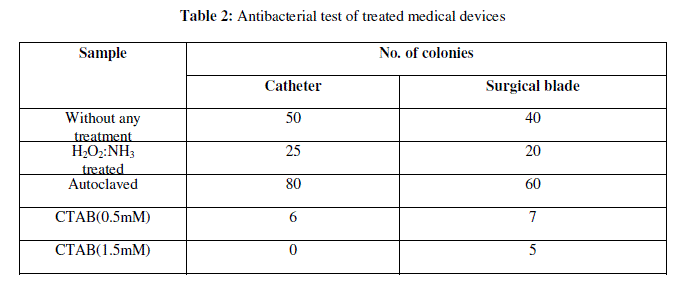
| The antibacterial activity of modified glass surfaces was determined by comparing the number of bacterial colonies of Staphylococcus spp., E. coli and Pseudomonas spp. grown on coated test surfaces relative to plain glass slides. Table 1a to 1c shows the mean value of bacterial colonies of E. coli, Pseudomonas spp. and Staphylococcus spp., grown on plain glass slides after 1day, 2days and 15days incubation respectively. Meanwhile, zero bacterial colonies if any were grown on coated surfaces with CTAB (0.5 and 1.5mM), which reflected high antibacterial activity. One and two colonies were observed after 15 days incubation. |
 |
 |
 |
C. Characterization of the glass slides after Plasma treatment and CTAB coating: |
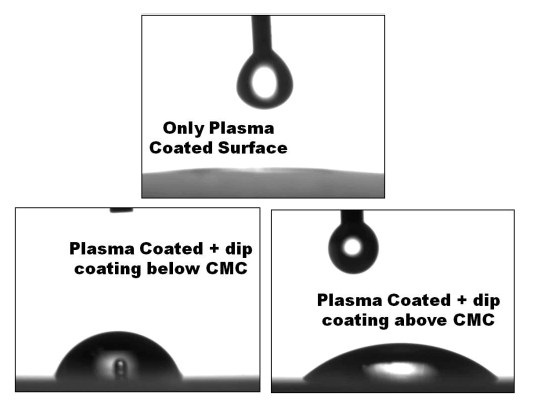
| The results obtained from Goniometer test for wettability is shown in Figure 2. It clearly indicates when dipped at a concentration above CMC the surface is hydrophilic and vice versa. These properties are very important for designing biomimetic antifouling surfaces. Detailed studies with Plasma modified surfaces are currently carried out in our lab. |
 |
IV. CONCLUSIONS |
| Biomimetic approach towards designing antifouling surfaces is our interest in making of this work. Most of the biological interactions that create self-assembled structures originate from electrostatic interactions. Hence, the chemicals selected for coating surfaces were mostly ammonium type molecules which are cationic and also reported to have antibacterial properties8. Examples can be cited from coral reefs under the sea. Even polymers having ammonium groups as side chain functional groups have been reported in literature to show antifouling properties8.Among the samples tested, CTAB showed best results. Tests were also done with field collected samples. Medical devices like catheter and surgical blade were coated with CTAB and checked for bacterial infection. CTAB shows antibacterial property might be due to the presence of ammonium group in its molecular structure. Thus results of these studies showed that CTAB can be used to design the antifouling surfaces and for sterilization for medical devices.The above process can be accepted as a general method by which any kind of surface can be rendered benign or antifouling. Further studies are on to test the effect of these surfaces on other species as well as on the environment. |
References |
|