ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Binay Bhushan1, Asima Pradhan2
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
We have employed Raman Spectroscopy for the diagnosis of breast and cervical cancer. A temperaturestabilized diode laser (Starbright, Torsana Laser Technologies) operating at 785 nm was used to record the Raman spectra of breast and cervical tissues. All the spectra were background corrected by the LabSpec software before being filtered using the commercial software Microcal Origin by taking 50 points. The differentiation between the normal and malignant tissues was investigated on the detection algorithms based on the ratio of peak intensities of representative bands. Based on the limited number of tissues of patients available to us, we got a decent percentage of sensitivity as well as specificity for the diagnosis of breast and cervical cancer.
Keywords |
| Raman Spectroscopy, Cancer, Tissues, Laser. |
INTRODUCTION |
| Cancer has been one of the deadliest diseases since ages. Its early detection and subsequent prevention from proliferation in human body have been a challenging task for researchers all over the world. Some of the conventional techniques used for the diagnosis of cancer are mammography, ultrasonography, magnetic resonance imaging (MRI), positron emission tomography (PET) and histopathology. However, these techniques have limitations in the sense that these are slow, invasive and expensive. Moreover, the images obtained with these techniques offer limited information regarding the screened region and they do not offer sufficient evidence of the grade of malignancy of the tumour. To gain better knowledge of the state of malignancy, a biopsy is required, and the assessment of biopsy tissues and subsequent diagnosis of cancer depend on the experience of the pathologist. Therefore, alternative techniques for clinical diagnosis of cancer should be explored to reduce the subjectivity of human error. In order to overcome all these limitations, researchers have been looking for light based optical techniques which can be noninvasive (or minimally invasive) and cost effective as well. Among the optical techniques, spectroscopy has emerged as powerful techniques for the early and quick diagnosis of cancer. For the last three decades, several noninvasive spectroscopic techniques such as Raman, infrared, and fluorescence have been used for the early detection of cancer in human tissues. Emerging as a frontrunner among the optical diagnostic technologies is Raman spectroscopy. This technique offers the possibility of nonintrusive diagnostics, both in vitro as well as in vivo and provides a detailed biochemical composition of the sample. This is a laser-based inelastic scattering spectroscopic technique and refers to the scattered light from a molecule or cellular sample which exhibits a frequency shift that reflects the energy of specific molecular vibrations within the sample of interest. This manifests itself physically as a unique shift in the wavelength of the photon away from the incident wavelength. In this manner, it provides a detailed biochemical composition of the sample - a biochemical fingerprint in essence. The collection and examination of these shifted wavelengths, unique to their scattering bond, result in the construction of Raman spectrum which is a plot of energy shift away from the incident wavelength, usually measured in relative wavenumbers versus scattered intensity. Raman spectroscopy offers the possibility of objectively characterizing a variety of clinical samples based upon the biochemical changes associated with the development of neoplasia. This technique requires very little sample preparation and is compatible with endoscopic approaches. Here we present our preliminary study based on the Raman spectroscopic technique to differentiate amongst the normal and abnormal counterparts of human breast and cervical tissues. |
II. EXPERIMENTAL TECHNIQUE AND METHOD |
| A temperature-stabilized diode laser (Starbright, Torsana Laser Technologies) operating at 785 nm was expanded and passed to the sample via a 10X objective. The backscattered Raman light was collected by the same objective and then imaged onto the SPEX 1877 triple spectrometer equipped with a liquid nitrogen cooled CCD. The setup was first calibrated with diamond for which the Raman signal (at 1332 cm-1) has been shown in the Fig. 1. The resolution of the system was 4 cm-1. |
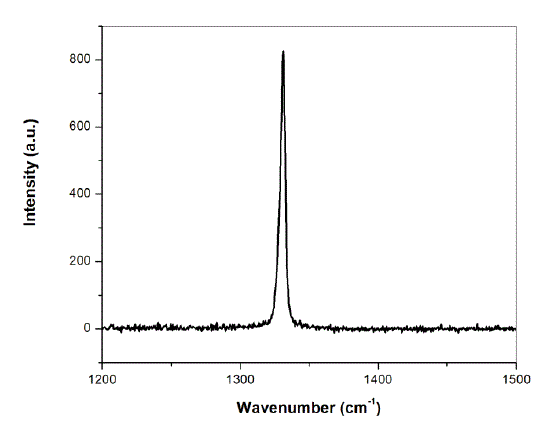 |
| Fig.1: Raman spectrum of diamond recorded for the calibration of Raman set-up |
| The laser power at the site of the sample during signal acquisition was 15 mW while the integration time was 120 sec. It has been shown that the „fingerprint regionâÃâ¬ÃŸ which contains the majority of the useful biochemical information obtained by Raman spectroscopy for most of the tissues is ~ 800 – 1800 cm-1. However, because of superimposition of diode laser wavelength over a broad pedestal of UV-Visible region which gives a large fluorescence background, going below 1200 cm-1 was not successful. In order to get rid of this problem, a laser line filter (10LF20-780, Newport) was employed to filter out the 785 nm laser wavelength, but because of only ~ 50 % transmission of filter around 785 nm, good quality spectra were not obtained. Therefore, recording the spectra of cervical and breast tissues were restricted to the region 1200-2400 cm-1 and 1200-2200 cm-1 respectively to avoid the large fluorescence background. This region was found to be sufficient to discriminate amongst the normal and abnormal counterparts of respective tissues. Our study contained two groups: 7 patients of breast cancer and 12 patients of cervical cancer with their normal counterparts. Raman spectra were recorded for normal as well as abnormal counterparts of respective tissues. All the spectra were background corrected by the LabSpec software before being filtered using the commercial software Microcal Origin by taking 50 points. For collecting the signal in the backscattering mode, flatness of the tissue surface is of utmost importance, because even a slight tilt of the surface will render the scattered signal away from the beam collecting diameter of the objective. Hence, the tissues were squeezed between two quartz slides in order to ensure the flatness of their surfaces. Because of its excellent transmission in the range of wavelength of our interest, the quartz slide does not affect the |
III. RESULTS AND DISCUSSION |
| A. Raman spectra of breast tissues Fig. 2 shows the background corrected, filtered and normalized Raman spectra of normal and abnormal counterparts of breast cancer patient. The spectra were normalized with respect to peak intensity in order to facilitate the comparison between the normal and abnormal tissues. |
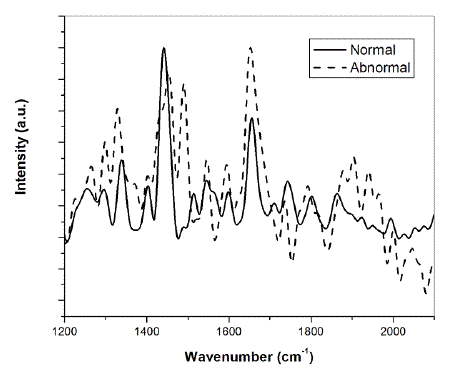 |
| Fig. 2: Raman spectra of normal and abnormal breast tissue |
| A careful examination of the two spectra shows the shifting of some of the peaks. It has been reported earlier that some peaks in the malignant breast tissue are shifted when compared to their benign counterparts [1]. Moreover, some of the peaks show differences in intensity. Specifically, one can easily detect the differences in relative intensity between I1441 and I1652 where I corresponds to the intensity of the band and the sub-index to the position of the band respectively. The band at 1441 cm-1 is assigned to phospholipids, CH scissor in CH2 while that at 1652 cm-1 corresponds to proteins and phospholipids [1]. |
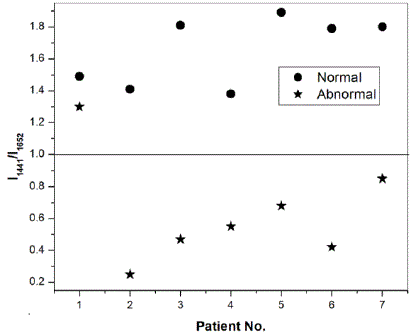 |
| Fig. 3: Intensity ratio at peak positions 1441 cm-1 and 1652 cm-1 for breast cancer patients |
| Fig. 3 shows the plot of the ratio of the intensities of the two peaks for the normal and abnormal counterparts of seven patients on which preliminary studies were performed. It is obvious from Fig. 3 that for all the seven patients suffering from breast cancer, the intensity ratio for normal tissues is greater than 1, while for all but one abnormal tissues, this ratio is less than one. Specifically, we have found the average characteristic value of the ratio for normal tissues to be 1.65 ïÃâñ 0.22 while that for abnormal tissues, this ratio is 0.65ïÃâñ0.35 . Alfano et al. have also reported such characteristic ratio of benign tissues, benign tumours and malignant tissues [2]. Other research groups have also studied cancer of colon, lungs and cervix on the basis of this method [3-5]. Our preliminary studies on seven breast cancer patients following this approach have resulted in 86% sensitivity and 100% specificity. We are of the opinion that a proper statistics could be performed after experimentally investigating the Raman spectra of a large number of patients, instead of what we did on only seven patients which were available to us. B. Raman spectra of cervical tissues Raman spectra were recorded from normal and abnormal human cervical tissues in vitro. The differentiation between the two types of the tissues was investigated on the detection algorithms based on the ratio of peak intensities of representative bands similar to reported above. Fig. 4 shows the background corrected, filtered and normalized Raman spectra of normal and abnormal counterparts of cervical cancer patient. The spectra were again normalized with respect to peak intensity in order to facilitate the comparison between the normal and abnormal tissues. |
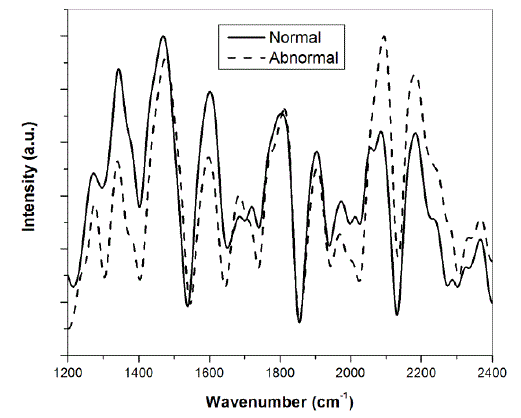 |
| Fig. 4: Raman spectra of normal and abnormal cervical tissue |
| The characteristic peaks occurring at 1308, 1340, 1452, 1600 and 1661 cm-1 have been assigned to CH2 deformation in lipids/adenine/cytosine, polynucleotide chain (DNA bases), CH2 deformation in lipids, C=C bending in phenylalanine and tyrosine and AmideI:alpha-helix respectively [6]. In some of the malignant tissues, the band at 1600 cm-1 was found to be shifted on the higher wavenumber side. The shifting of this band has also been reported in the literature [7]. We have differentiated the normal and abnormal counterparts of the cervical tissue based on the ratio of intensity of band occurring at 1452 cm-1 and 1600 cm-1. Based on our preliminary study on 12 cervical cancer patients, we found that for both the normal and abnormal counterparts of the cervical tissues, the intensity of band at 1452 cm-1 is greater than that at 1600 cm-1. This was found to be contrary in only one for normal and two for abnormal tissues, out of a total of 12 patients. Specifically, the average characteristic value of the ratio of intensity of these two respective peaks is 1.33 ïÃâñ 0.34 and 1.41ïÃâñ0.39 for normal and abnormal tissues. |
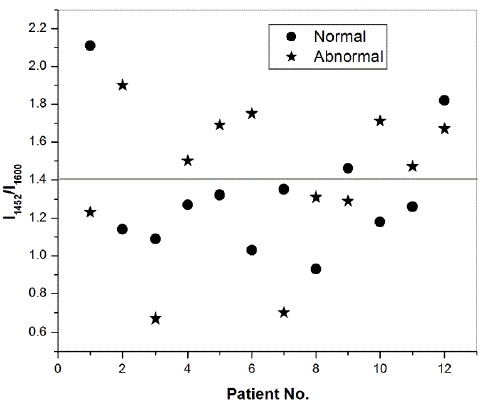 |
| Fig. 5: Intensity ratio at peak positions 1452 cm-1 and 1600 cm-1 for cervical cancer patients |
| However, the differentiation between the two types of tissues can be done on the basis of comparing the ratio of the intensities of these two respective peaks individually for the normal and abnormal counterparts of different patients. We found that out of 12 patients, this ratio is greater for 7 abnormal tissues and for 5 normal tissues, as shown in Fig. 5, resulting in 67% of sensitivity as well as specificity. Such a differential scheme has also been reported in the literature [7]. Moreover, we have found that, as shown in Fig. 4, that the peak intensity at 2065 cm-1 and 2191 cm-1 for abnormal tissues is more than their normal counterparts. This we have observed for 7 out of a total 12 tissues (success rate 58 %). We conclude that a careful study on a large number of samples can yield better sensitivity as well as specificity. Such a study can also represent a demarcation line for the ratio of these intensity peaks as we have drawn in Fig. 5, where out of 12 patients, the ratio for abnormal tissue is greater than 1.4 for seven out of total twelve patients (success rate of 58%, bound to improve for a large number of samples). We have shown above in Fig. 3 that such a demarcation line manifests perfectly for the differentiation of normal and abnormal tissues in case of breast cancer patients. |
IV. CONCLUSIONS |
| We have used Raman Spectroscopy for the diagnosis of breast and cervical cancer. A temperature-stabilized diode laser (Starbright, Torsana Laser Technologies) operating at 785 nm was used to record the Raman spectra of breast and cervical tissues. All the spectra were background corrected by the LabSpec software before being filtered using the commercial software Microcal Origin by taking 50 points. The differentiation between the normal and malignant tissues was investigated on the detection algorithms based on the ratio of peak intensities of representative bands. Based on the limited number of tissues of patients available to us, we got a decent percentage of sensitivity as well as specificity for the diagnosis of breast and cervical cancer. We conclude that a careful study on a large number of samples can yield better sensitivity as well as specificity. |
ACKNOWLEDGMENT |
| The corresponding author is grateful to Council of Scientific and Industrial Research (CSIR), Govt. of India for providing the financial support for this research work. |
References |
|