ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Alkesh Hada1, Amit Kumar Gupta1, Theboral Jeevaraj2, Markandan Manickavasagam2, Andy Ganapathi2, Monica Jolly1 , Archana Sachdev1
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Regeneration through mature cotyledonary node has set rapid regeneration of plants directly from explants which is more time-saving and presented as an effective strategy so we have developed regeneration protocol through single shoot using cotyledonary node a rapid and efficient protocol for three Indian soybean cultivars. Two explants were collected from single cotyledonary node and cultured on medium containing N6-benzylaminopurine (BAP) for germination, BAP and indole-3-butyric acid (IBA) for shoot induction, Gibberellic acid (GA3) for shoot elongation and IBA for rooting of explants. The best combination of hormones for all genotypes were obtained as germination of seeds on half B5 medium supplemented with 1.0 mgl-1 of BAP, shoot induction on full B5 medium having BAP 1.0 mgl-1 and IBA 0.2 mgl-1 and shoot elongation on GA3 0.75 mgl-1 in full MS medium. Under these conditions, the plantlets could be raised within 40-45 days. It was observed that selection of proper medium for regeneration of soybean can overcome genotype associated problems. This regeneration system can be used for soybean transformation
Keywords |
| Soybean genotype, transformation, regeneration, cotyledonary node. |
Abbreviations |
| BAP – 6-benzyl-aminopurine, IBA - Indolebutyric acid, GA3 - Gibberellic acid, MS - Murashige and Skoog. |
INTRODUCTION |
| Soybean [Glycine max (L.) Merrill] is widely used as oil and protein source for human, as livestock feed. It is also a source for plastic, adhesive as well as in a variety of items of processed food in industries. It contains 40% of protein and 20% of oil, which is the highest protein among the pulses. In soybean production, USA is at the first position with the annual production of about 80.7 million metric tons followed by Brazil, Argentina and China. The annual production of soybean in India is 10.12 million metric tons (FAOSTAT 2009). |
| The major constrain in soybean production are susceptibility to pathogens and pests, environmental stresses, poor pollination and low harvest index. Traditional breeders have made effort in the development of new cultivars of soybean for disease, pest and herbicide resistance; and increased nutritional value. But, traditional breeding programmes are having limitation because soybean germplasm is extremely narrow and the majority of the soybean cultivars in use are derived from very few parental lines (Christou et al. 1990). Serkan et al. (2005) and Haliloglu (2006) reported that on the basis of efficient plant regeneration protocol, biotechnology can be apply successfully in crop improvement. |
| Legumes are the most recalcitrant to in vitro manipulation but with great interest routine protocols are obtained for stable transformants for the major grain legumes such as the common bean (Phaseolus vulgaris), soybean (Glycine max), pea (Pisum sativum), peanut (Arachis hypogea), and alfalfa (Medicago sativa), as well as the model legume, barrel medic (Medicago truncata) (Christou 1992; Puonti-Kaerlas et al. 1990; Russell et al. 1993). To date many types of primary explants have been used for soybean regeneration via direct organogenesis. These primary explants include cotyledonary node (Cheng et al. 1980; Barwale et al. 1986; Hinchee et al. 1988; Wright et al. 1986; Shetty et al. 1992; Kaneda et al. 1997; Shan et al. 2005), stem-node (Saka et al. 1980; Kim et al. 1990), primary leaf tissue (Wright et al. 1987a), epicotyl sections (Wright et al. 1987b), cotyledons (Mante et al. 1989; Franklin et al. 2004), plumules (Yang et al. 1990), hypocotyls (Kaneda et al. 1997; Dan and Reichert 1998; Yoshida 2002) and embryonic axes (McCabe et al. 1988; Liu et al. 2004). Regeneration through mature cotyledonary node has set rapid regeneration of plants directly from explants which is more time-saving and presented as an effective strategy. In general, soybean tissue culture is not only time consuming but also genotype dependent (Franklin et al., 2004). Each method has limitation for the production of transgenic plants and the regeneration protocol does not seem high enough for soybean transformation. Therefore, an improvement in the regeneration would contribute to an increase in the production of transgenic soybean. An efficient protocol on regeneration of different Indian soybean cultivars is reported in this study. |
MATERIAL AND METHODS |
| Plant material: |
| Three Indian soybean cv. JS 335, JS 95-60 and NRC 37 were used to standardize the regeneration protocol with various parameters. The genotypes were obtained from Directorate of Soybean Research (DOSR), Indore, Madhya Pradesh, India. |
| Basal media and culture conditions |
| The medium used in this study was MS (Murashige and Skoog et al., 1962) and B5 (Gamborg et al., 1968) supplemented with various concentrations and combinations of plant growth regulators. The media were supplemented with 3% sucrose and were solidified with 0.6% agar, adjusted to pH 5.8 with 1N NaOH/1N HCL then autoclaved at 121-123°C for 20 min before using. The tissue culture room was maintained at 26±2°C under a light-dark cycle of 16:8 with a light intensity of 60 μmol m-2s-1. |
| Explant preparation and regeneration |
| Dry, mature seeds of all three varieties were sterilized by treating seeds with chlorine gas made by mixing 3.5 ml of 12 N HCL and 100 ml bleach (4% sodium hypochlorite) for 5-6 hr (Di et al., 1996). Fifty sterilized seeds were placed in germination medium (GM) (1/2 strength B5 supplemented with 3% sucrose, 0.6% agar and pH 5.8) supplemented with various concentration of N6-benzylaminopurine (BAP) (0 mgl-1, 1 mgl-1, 2 mgl-1, 3 mgl-1, 4 mgl-1 and 5 mgl-1). The planted seeds were kept in a tissue culture chamber at 26±2°C under cool white fluorescent lights (90-150 μmol photons m-2 s-1) in a 18/6 h (light/dark) photoperiod for 5-6 days, or until the cotyledons become green and seed coat split open, but before the first leaves expanded to the length of the cotyledons (Olhoft et al. 2003). |
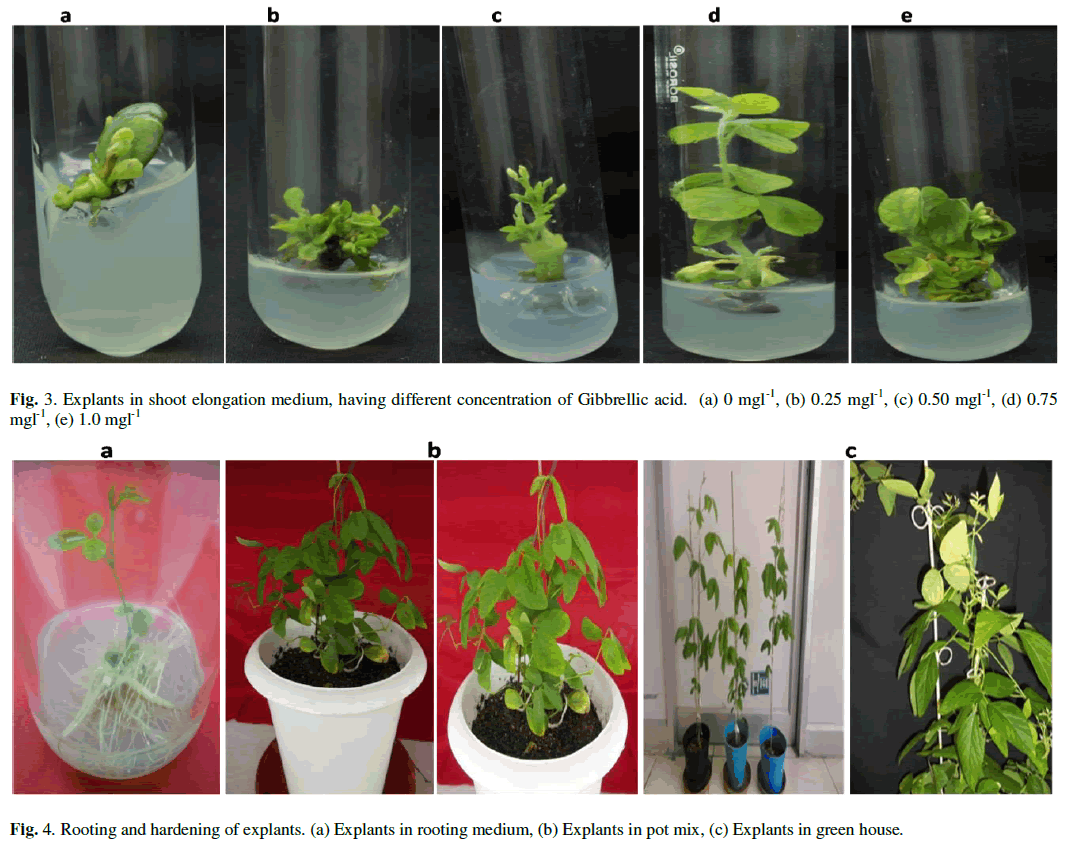
| Roots and the major portion of the hypocotyls approx. 3-5 mm below the cotyledonary node on the hypocotyls were removed, separating the cotyledons. A vertical cut through the remaining hypocotyls was made with a surgical blade. The epicotyl was subsequently removed and 100 such explants were placed on shoot induction medium (SIM) (full strength B5 medium supplemented with 3% sucrose, 0.6% agar and pH 5.8) having constant concentration of BAP (1 mgl-1) and different concentration of indole-3-butyric acid (IBA) (0 mgl-1, 0.2 mgl-1, 0.5 mgl-1 and 1 mgl-1). The explants were kept for 10-12 days under above mentioned culture conditions, After 12 days, the cotyledonary node were trimmed 1/3 from the explants and the explants with newly developed shoots were transferred to the shoot elongation medium (SEM) (full MS medium supplemented with 3% sucrose, 0.6% agar and pH 5.8) with different concentrations of Gibberellic acid (GA3) (0 mgl-1, 0.25 mgl-1, 0.50 mgl-1, 0.75 mgl-1 and 1.0 mgl-1). The explants were sub cultured to fresh SEM medium until the shoots elongated 4-5 cm in length. When the shoot length reached 4-5 cm, the newly developed shoots were placed in rooting medium (1/2 B5 medium supplemented with 3% sucrose, IBA 2 mgl-1, 0.6% agar, and pH 5.8). Explants remained in the same rooting medium throughout the rooting. The roots were formed in 15-20 days and rooted plantlets were hardened into potmix on tissue culture condition. Then after 10-12 days it shifted to National phytotron facility, IARI, New Delhi for maturation under a 16/8 h (light/dark) photoperiod and natural light supplemented with 1,000-W high-pressure sodium lamp. |
| Experimental design and statistical analysis |
| Four factors including BAP, IBA, GA3 and soybean genotypes were studied with reference to the above described regeneration protocol. The experiment was repeated three times to examine influence of each factor on efficiency of the regeneration protocol. Data were analyzed using one-way analysis of variance (ANOVA). The mean value of the treatments was analyzed using Duncan Multiple Range Test (DMRT). |
EXPERIMENTAL RESULTS |
| Effects of hormones on seed germination and shoot regeneration |
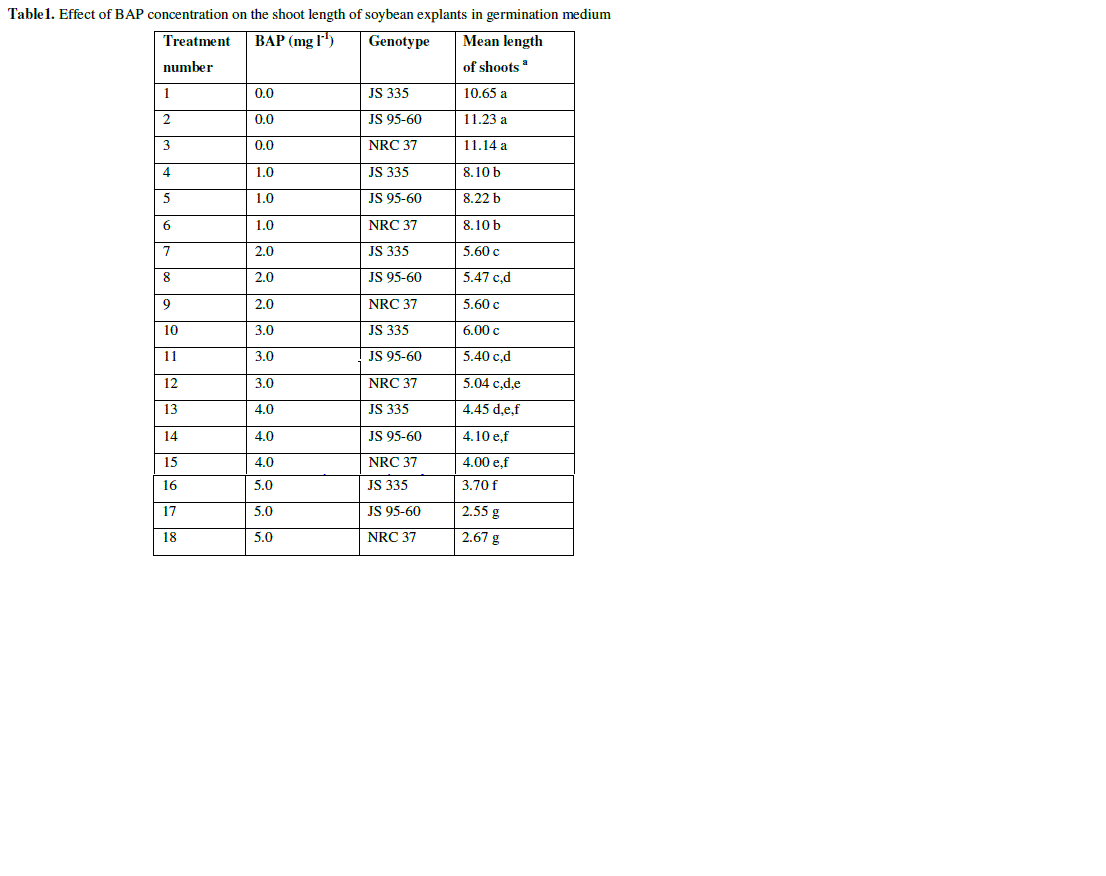
| Seeds of three cultivars were germinated on half B5 medium for 5-6 days. The medium was supplemented with BAP at different concentration (1 mgl-1, 2 mgl-1, 3 mgl-1, 4 mgl-1, 5 mgl-1), in which the germination frequency was 97.2, 98.8, 98.9, 98.7, 98.7 and 98.6% respectively (data not shown). In control medium (in the absence of BAP) seedlings germinated had dark green cotyledon, thin and longer hypocotyl and lateral roots. The seedlings which germinated on 1 mgl-1 of BAP had 8.1 cm length on average with green cotyledon and without any lateral roots. Length of the seedlings decreased gradually with the increase in hormone concentration (Fig. 1 and Table 1). |
| Table1. Effect of BAP concentration on the shoot length of soybean explants in germination medium |
 |
 |
| a The mean value were calculated from three replicates in each treatment and each replicate was represented by five plants |
| Values with the same letter are not significantly different according to Duncan’s Multiple Range Test (DMRT) at 5% level |
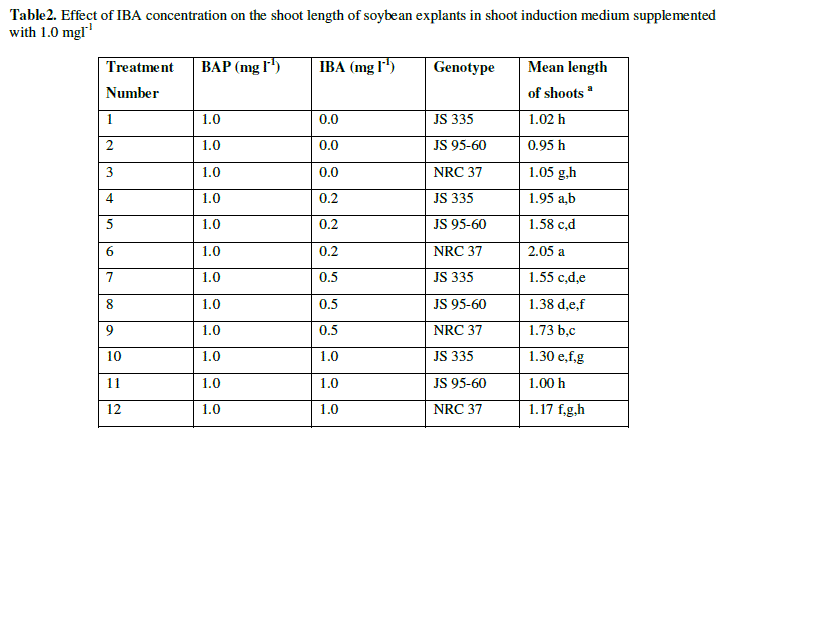
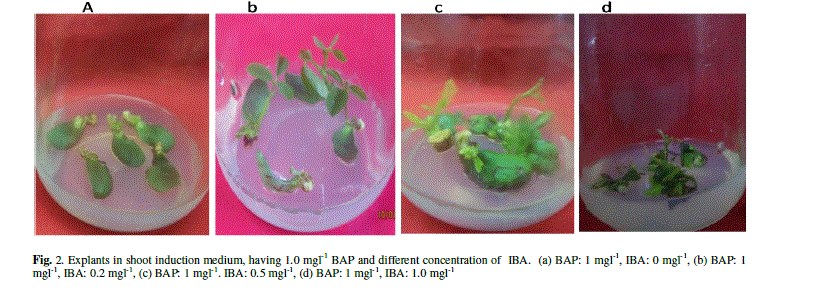
| The explants were transferred on SIM to induce the shoots with constant concentration of BAP (1 mgl-1) and various concentration of IBA (0 mgl-1, 0.2 mgl-1, 0.5 mgl-1, 1 mgl-1). The data were recorded 10 days after incubation on SIM. All of them produced adventitious multiple shoots. There were no obvious differences on regeneration frequency between the explants with different combination of hormones (96.6, 97.2, 97.6 and 97.5%) (Data not shown). Longest shoots obtained with an average length of 1.86 cm with the combination of BAP (1 mgl-1) and IBA (0.2 mgl-1). But, length of the shoots decreased and browning of the contact surface of the cotyledonary node and hypocotyl increased gradually with the increase of IBA concentration (Fig. 2 and Table 2). |
| Table2. Effect of IBA concentration on the shoot length of soybean explants in shoot induction medium supplemented with 1.0 mgl-1 |
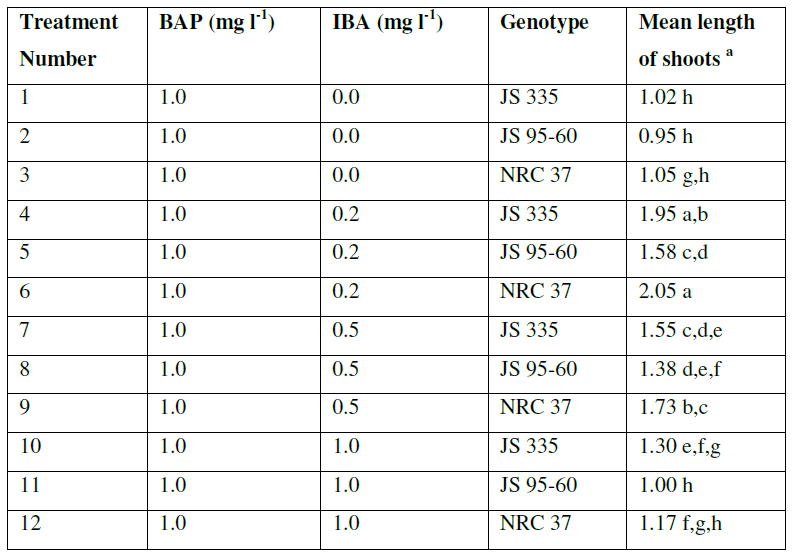 |
| a The mean value were calculated from three replicates in each treatment and each replicate was represented by five plants |
| Values with the same letter are not significantly different according to Duncan’s Multiple Range Test (DMRT) at 5% level |
| After 10 days in SIM, the explants were placed in SEM with different concentrations of Gibbrellic acid (GA3) (0 mgl-1, 0.25 mgl-1, 0.50 mgl-1, 0.75 mgl-1 g/l and 1.0 mgl-1). Length of the shoots was measured after 20 days on SEM. Shoots elongated at all concentrations of GA3. Length of shoots gradually increased up to 0.75 mgl-1 of GA3 concentration on average 4.3 cm. But, length decreased (3.20 cm) at 1.0 mgl-1 of GA3 (Fig. 3 and Table 3). |
| Table3. Effect of GA3 concentration on the shoot length of soybean explants in shoot elongation medium |
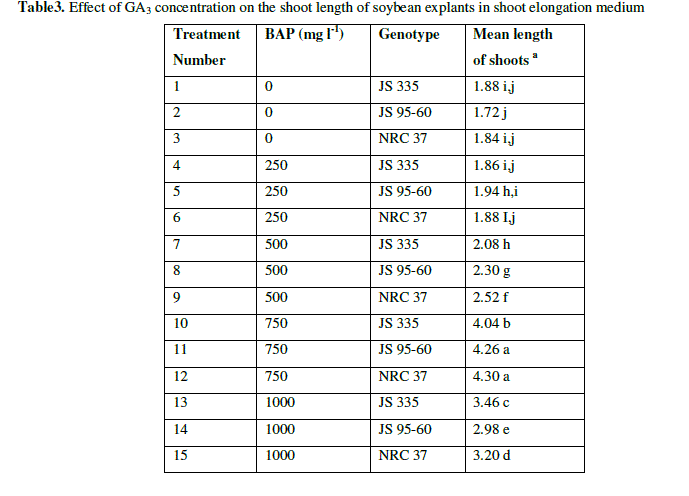 |
| a The mean value were calculated from three replicates in each treatment and each replicate was represented by five plants |
| Values with the same letter are not significantly different according to Duncan’s Multiple Range Test (DMRT) at 5% level |
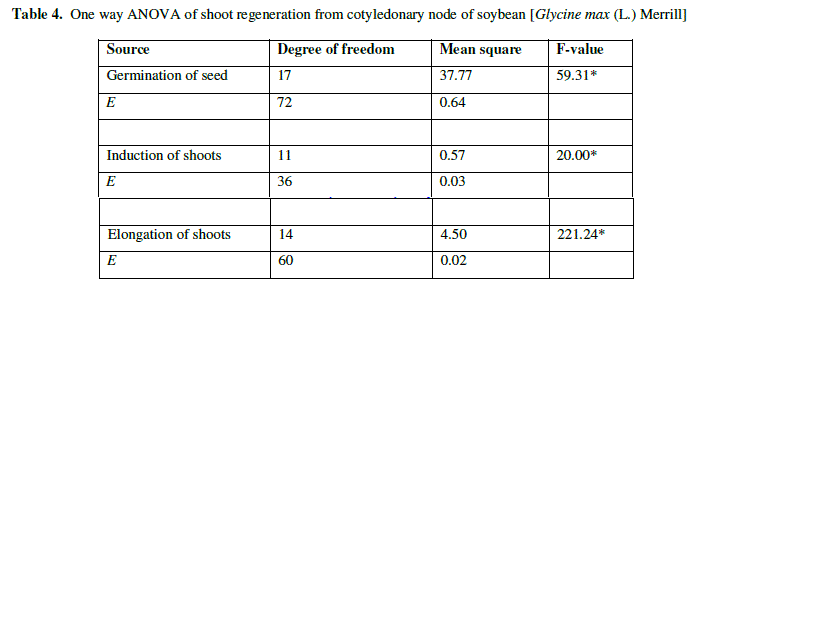
| The ANOVA table showed that results are significant on single shoot regeneration of mature cotyledonary node (P<0.01) (Table 4). The length of shoots was maximum when the explants were kept in optimum concentration of hormones. |
| Table 4. One way ANOVA of shoot regeneration from cotyledonary node of soybean [Glycine max (L.) Merrill] |
 |
 |
| e Experimental error |
| * Level of difference at P<0.01 |
| Comparisons of different hormones and genotypes |
| The results showed obvious difference in response to shoot regeneration for the various combinations of hormones. Single shoot was regenerated on SIM in all the combination of hormones. Whereas, on the 2nd treatment i.e., SEM supplemented with various concentration of GA3, single shoot elongated. The results summarized in Tables (1, 2, 3) illustrate the best combination of the hormones to regenerate single shoot. In Table 1, results showed the best combination for germination on MS medium supplemented with 1 mgl-1 of BAP for all genotypes. In Table 2, it is evident that the best combination for induction of single shoot is the medium supplemented with 1 mgl-1 BAP and 0.2 mgl-1 IBA for all genotypes. From the data in Table 3, it is clear that the medium supplemented with 0.75 mgl-1 of GA3 was found to be best for elongation of shoots for all genotypes. |
 |
 |
| Comparisons among genotypes |
| The efficiency of genotypes (JS 335, JS 95-60 and NRC 37) was examined to determine the effect on shoot regeneration. These genotypes had no effect on regeneration efficiency. |
| Duration of regeneration using cotyledonary nodes |
| After sterilization, seeds were transferred on GM for 5-6 days. Two explants were excised from each seedling and placed to SIM. After 10-12 days, single shoot buds emerged which could regenerate directly from cot node without a callus stage. Hereafter, induced single shoot was transferred on SEM to 3-4 cm long in 20-24 days. Most of the elongated shoots rooted on rooting medium in just 10-15 days. The whole in vitro regeneration period was completed in 40-50 days. The rooted plants were hardened in pot mix and then transferred to the National phytotron facility to mature. |
DISCUSSION |
| Researchers have proved that cotyledonary node is good candidate explant for shoot regeneration (Hinchee et al. 1988; Oholf et al. 2007). The present study has some advantages over other method. First, this system takes less time than other explants used. The whole process of regeneration is shortened to 40-45 days. Secondly, using cotyledonary node as explant may lead fewer calluses on the medium supplemented with high concentration of BAP. Three Indian soybean cultivars were used in the present study to standardize the regeneration protocol, which showed that the protocol is genotype independent. |
| Germination medium were supplemented with the cytokinin to stimulate shooting. The seedling which germinated on medium without cytokinin showed abnormal shoots, therefore were not selected for study. The optimum concentration of BAP was observed to be 1.0 mgl-1 to produce healthy shoot in all the seeds. But, the shoot length started to decrease when the cytokinin concentration was further increased. This phenomenon is similar to a research on micropropagation of different banana cultivars, where the shoot length increased with higher BAP level until 22.2 μM after which the shoot length also began to fall (Shirani et al. 2010). Tang et al. (2012) also reported that, increased BAP concentration reduced shoot proliferation and increased the differentiation of abnormal shoots and suppressed the elongation of the shoots. |
| After standardization of GM, the cotyledonary node was kept in shoot induction medium to induce the shoots. The medium was provided with cytokinin (BAP) for cell division and auxin (IBA) for shoot induction. The shoot induction medium was supplemented with constant concentration of BAP (1 mgl-1, as standardized in GM) with various concentration of IBA. The desirable concentration for shoot induction was found 0.2 mgl-1 for all three genotypes. The shoot length decreased and become abnormal below and above to 0.2 mgl-1 of IBA concentration (Table 2). This finding is supported by observation of Shirani et al. (2010); Tang et al. (2012). |
| After 10-12 days, the induced shoots were placed in SEM to elongate shoots. The medium was supplemented with various concentrations of GA3. In this study, only GA3 was used for shoot elongation due to various reasons. First it is the most powerful growth promoters because they, increase internode spacing, second GA3 controls stem elongation by stimulating both cell division and elongation, third GA3 promotes uniform growth of shoot through cell enlargement and fourth, it stimulates plants to grow tall and elongate with light green leaves. Keeping the above points in mind, GA3 was used for shoot elongation in this study. The shoot elongation decreased below and above the 0.75 mgl-1 of GA3. This result showed that, increased concentration of GA3 reduced the shoot length and increased the abnormal shoots formation. Olhoft et al. (2003) have used various combinations of hormone for shoot elongation but, their time of regeneration was comparably longer. |
| This was first study of regeneration of three Indian soybean cultivars using cotyledonary node as explants. Some differences were observed in genotypes with regards to the shoot length, but it was acceptable (Table 2). It was observed that selection of proper medium for regeneration of soybean can overcome genotype associated problems. Overall, the regeneration period has been shortened to only 40-45 days, which is the shortest duration of soybean regeneration. This standardized regeneration system will be compatible with Agrobacterium-mediated soybean transformations, and after newer strategies for the control of pests and diseases. |
CONCLUSION |
| The demand of soybean oil and protein is increasing day by day, the improvement of soybean quality and production through genetic transformation becomes a relevant issue through out of world. The relevance of this study was to determine the optimum concentration of basal media and hormones for in improving the soybean regeneration and minimizing the duration of regeneration using cotyledonary-nodes. As well as comparisons of different Indian soybean cultivars is reported in this study. Therefore development of an efficient, simple, consistent transformation protocol for locally available commercial genotypes will greatly aid soybean transformation. |
ACKNOWLEDGEMENT |
| The financial support given by ICAR Government of India under NFBSFARA program is gratefully acknowledged. We are also grateful to the scientists and staff at National Phytotron Facility, Indian Agricultural Research Institute, New Delhi-110012, India, for their guidance and support to grow plants under controlled conditions. |
References |
|