Keywords
|
| Acyclovir, p-Toluenesulfonicacid, Method Validation, LC/MS/MS, Trace analysis |
INTRODUCTION
|
The chemical name for acyclovir is 2-amino-1,9-dihydro-9- [(2-hydroxyethoxy) methyl]-6H-purine-6-one, (or) 9- [(2-
hydroxyethoxy) methyl]- guanine. Its molecular formula is C8H11N5O3, and molecular weight 225.21 g/mol [1].
Acyclovir is a potent anti-viral agent useful in the treatment of Herpes Simplex Virus (HSV) infections. Acyclovir
exerts its antiviral activity by competitive inhibition of viral DNA, through selective binding of acyclovir to HSVthymidine
kinase with about 200 fold greater affinity than for mammalian enzyme [2]. |
Pharmaceutical genotoxic impurities (PGIs) may induce genetic mutations, chromosomal breaks (rearrangements) and
they have potential to cause cancer in human [3-4]. Therefore exposure to even low levels of such impurities present in
final active pharmaceutical ingredient (API) may be of significant toxicol importance [5]. Hence it is significant for
process chemists to avoid such genotoxic impurities in the manufacturing process [6]. However it would be difficult or
impossible to eliminate PGIs completely from the synthetic scheme. Therefore it is a great challenge to analytical
chemists to develop an appropriate analytical method to quantify the impurity accurately and control their levels in
APIs. According to the European Medicines Evaluation Agency (EMEA) and feedback from US Food and Drug
Administration (USFDA) the proposed use of a threshold of toxicological concern (TTC), it is accepted that genotoxic
impurities will be limited to a daily dose of 1.0-1.5 μg/day [7- 8]. |
Though p-Toluenesulfonicacid is a well known carcinogen, this data would ascertain that the regulatory authorities may
be expected to control the levels of p-Toluenesulfonicacid 1.5 ppm in the drug substance. p-Toluenesulfonic acid is
used as counter-ions for basic drugs during the synthesis of the drug substance in pharmaceutical industry because of its
strong acidic and hydrophilic properties [9-10] as well as the catalyst system is being an organic in character[11]. It has
broad application towards, oxidative degradation [12], transesterification of an ester [13], esterification of carboxylic
acid [14] and reductive amination of aldehydes and ketones [15] as well as reaction mediator [16]. The utilization of
alcohols from synthetic reaction or in the salt formation steps may result in the formation of corresponding alkyl tosylates, which are potential and known to be genotoxic impurities [17]. A method capable of such a lower level of
detection is great challenge for analytical method development for controlling these genotoxic impurities. It was
deemed necessary to develop an assay method for simultaneous quantification of p-Toluenesulfonicacid and acyclovir
in API by LC-MS/MS with negative mode. This liquid chromatography method was developed and validated for use
in bioavailability and bioequivalence studies. Some of the analytical methods have been reported for acyclovir such as
luorescence [18-20] direct UV [21-24] and HPLC–MS [25]. In that numerous HPLC methods have been reported for
estimation of acyclovir in pharmaceutical formulations has been reported [26-34]. Among chromatographic techniques;
the reversed-phase (RP) HPLC was widely used for the analysis. Present study involves development of LC-MS/MS
method using simple mobile phase which was sensitive and rapid for quantification of acyclovir in impurity as well as
subsequent validation of developed method according to ICH guide lines [35]. To the best of our knowledge no
published method is available for the simultaneous determination of p-Toluenesulfonicacid and acyclovir API using
LC-MS/MS. This method provides high degree of precision, accuracy, sensitivity and stability by simple liquid – liquid
extraction based on liquid chromatography separation and detection in negative mode by electro-spray tandem mass
spectrometry. |
The present study was undertaken to develop a sensitive and rapid LC/MS/MS method for the determination of p-
Toluenesulfonicacid in acyclovir API. Due to its higher selectivity and sensitivity LC/MS/MS has been adopted for
quantification of p-Toluenesulfonicacid in acyclovir is a potent anti-viral agent, which is useful in the treatment of
Herpes Simplex Virus (HSV) infections. |
MATERIALS AND METHODS
|
MATERIALS
|
Methanol of HPLC grade was purchased from Merck (Mumbai, India). Analytical grade ammonium acetate, HPLC
grade water were purchased from Merck, (Mumbai, India). Water used for the LC-MS/MS analysis was prepared from
Milli Q water purification system procured from Millipore (Bangalore, India). Reference substance of p-
Toluenesulfonicacid was obtained from Sigma- Aldrich (St. Louis,USA). |
Preparation of Stock and Standard Solutions
|
Primary stock solutions of p-Toluenesulfonicacid was prepared in 10mg/mL of impurities in 100ml of diluent. Further
dilution 0.001mg/mL with diluents further achieved on 0.000015mg/mL with diluent. Diluted final concentration 1.5
ppm to get working solutions for obtaining calibration curve. |
METHODS
|
Method development and optimization
|
Optimization of chromatographic conditions was performed, particularly the composition of mobile phase, through
several trials to achieve symmetric peak shapes of the analytes peaks, as well as short run time and low cost. Resolution
negative mode acyclovir was achieved by using methanol as an organic content in the mobile phase. Separation was
attempted using various combinations of methanol and buffer with varying contents of each component on different
columns like C18 and C8 of different makes like Hypersil BDS column and Symmetry columns. Finally Symmetry
column was found to give the best chromatographic resolution with a flow rate of 0.4 mL/min and total run time of 8
min. The p-Toluenesulfonicacid and acyclovir were eluted at 4.06 and 2.10 min with selected ion monitoring (SIM)
mode. The inclusion of 5mM ammonium acetate instead of pure water enhanced the response and improved the
reproducibility. |
INSTRUMENTATION
|
HPLC operating conditions
|
A Shimadzu LC-20 AD Series HPLC system (Shimadzu Corporation, Kyoto, Japan) was used to inject 15μL aliquots
of the processed samples on a Symmetry C-18 (150 X 4.6mm, 3.5μm), which was kept at 30±2°C temperature. The
mobile phase, a mixture of 5mM ammoniumacetate and Methanol (70:30 v/v) was filtered through a 0.45μm membrane
filter (Millipore, USA or equivalent), then degassed ultrasonically for 5 min and delivered at a flow rate of 0.4 mL/min
into the mass spectrometer electrospray ionization chamber. |
Mass spectrometry operating conditions
|
Quantitation was achieved with MS-MS detection using a Applied bio system (AB SCIEX) API-4000 mass
spectrometer (Foster City, CA, USA) equipped with Turboionspray ™ interface at 400ºC. The MS/MS method consists
of negative mode. The ion spray voltage was set at -4500 V. The source parameters viz., the ion source gases GS1, GS2
and Nebulizer gas were set at 35, 30, and 13 psi respectively. The compound parameter viz. the declustering potential
(DP) and entrance potential were set at -53 and -10V. The selected ion monitoring (SIM) mode was used as MS method
for quantification of p-Toluenesulfonicacid in acyclovir drug substance. In this method p-Toluenesulfonicacid was
monitored with its molecular ion [M-H]+ m/z 171.2 (deprotonated) and acyclovir was monitored with its molecular ion
[M-H]+ m/z 224.2 (deprotonated). The analytical data obtained were processed by Analyst software™ (version 1.5.1). |
RESULTS AND DISCUSSION
|
METHOD VALIDATION
|
Specificity and selectivity
|
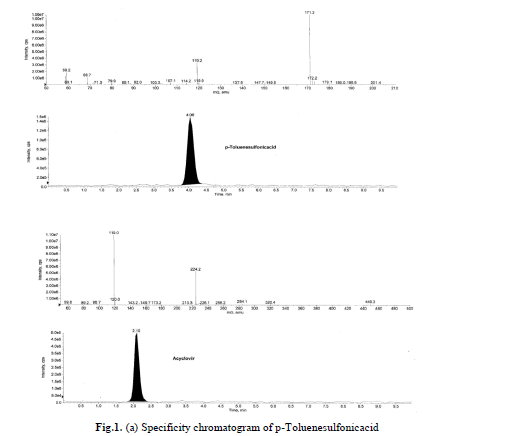
Specificity is the ability of the method to assess unequivocally the analyte response in presence of components that may
be expected to be present in the sample. Acyclovir and p-Toluenesulfonicacid compounds solutions were prepared
individually at a concentration of about 0.01mg/mL in the diluents and a solution of acyclovir spiked with p-
Toluenesulfonicacid were also prepared. Specificity was established by injecting acyclovir spiked with its impurity
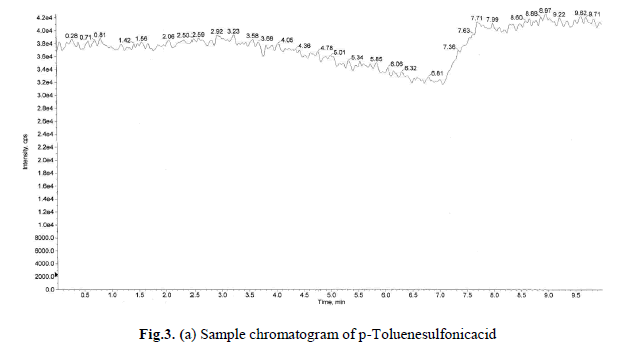
where in no interference was observed. Blank and specificity chromatograms are shown in Fig.1(a), (b). |
 |
 |
Robustness
|
The robustness of the developed method was studied with slight and deliberate changes in experimental conditions. The
effect of changes in flow rate of mobile phase (-2% to +2%) while the amounts of the other mobile phase components
were held constant, column oven temperature (-2°C to +2°C).i.e at 28ºC and 32ºC buffer units was studied. |
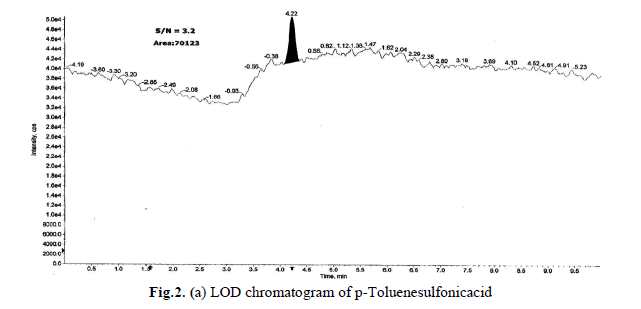
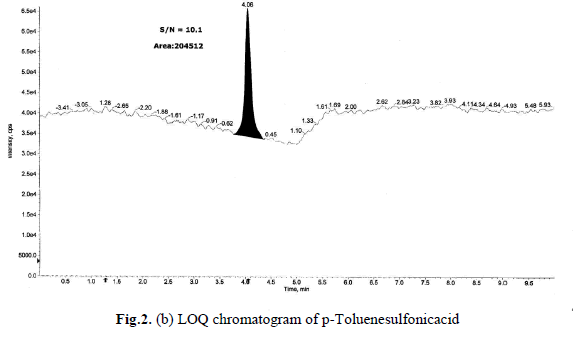
Determination of LOD and LOQ
|
The LOD and LOQ, as a measure of method sensitivity, were calculated from S/N (signal to noise) ratios. To determine
LOD and LOQ values for a p-Toluenesulfonicacid concentration were reduced sequentially such that they yield S/N
ratio as 3.2 and 10.1 respectively. The determined LOD and LOQ chromatograms were shown in Fig.2(a),(b).Data
generated from six injections of (without API) containing 1.5 ppm of each p-Toluenesulfonicacid with respect to an
API sample concentration 10 mg/mL. The LOQ of 1.5 ppm is typical for the p-Toluenesulfonicacid, with a LOD
approximately three times less than LOQ. In addition, the relative efficiency of SIM modes in sensitivity improvement
was also evaluated. |
 |
 |
Recovery studies
|
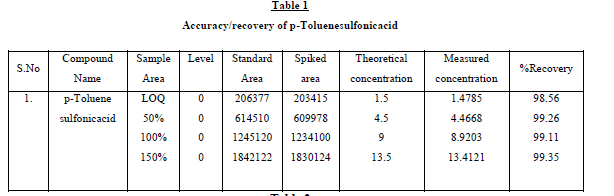
The recovery studies by the standard addition method were performed to evaluate accuracy and specificity, accordingly
the accuracy of the method was determined in triplicate at LOQ level in bulk drug sample. The recoveries were
calculated. Excellent recovery values of p-Toluenesulfonicacid 98.56 - 99.35 percentage were obtained. At such a low
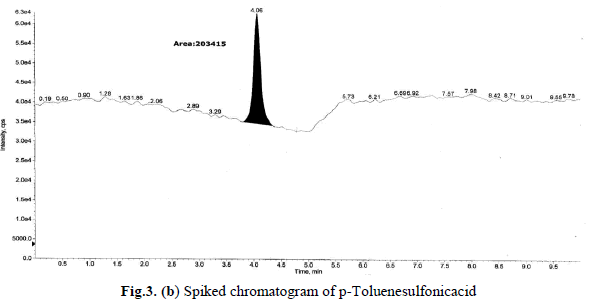
levels these recoveries and %RSD is <1.0 was satisfactory. Sample and accuracy at LOQ chromatograms are shown in
Fig.3(a),(b), and the relative standard deviation, %RSD were calculated from the average of triplicate analysis, which
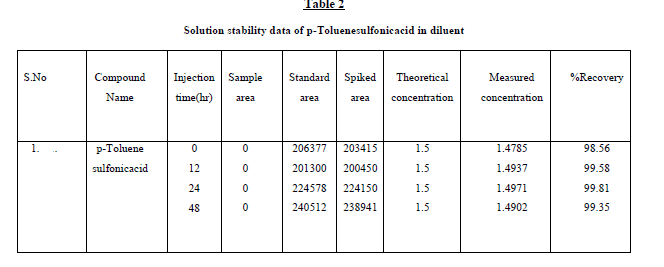
were shown in Table1. Further, the stability of p-Toluenesulfonicacid was found as 48 hr and the stability of this
impurity at different time intervals is presented in Table 2. |
 |
 |
 |
 |
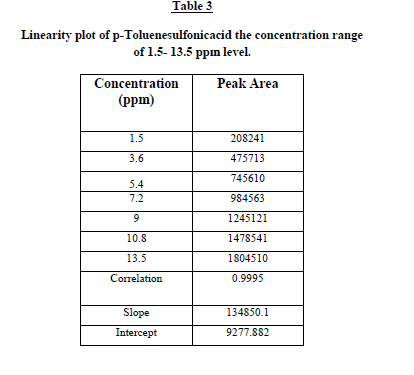
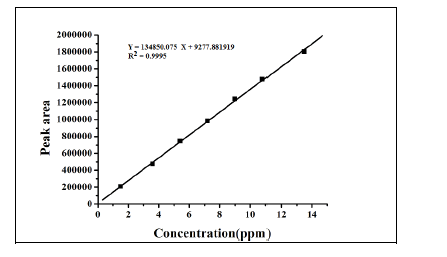
Linearity and range
|
The linearity test for the method was performed according to the guidelines laid by ICH. This method was evaluated at
six different concentrations of analytes with in the range of 1.5 – 13.5 ng/mL. These standard solutions were prepared
by suitable dilution of stock solution with mobile phase. The linearity of the plot was evaluated using least squares
linear regression analysis by selected ion monitoring (SIM). The linearity of p-Toluenesulfonicacid was satisfactorily
established with a six point calibration curve between LOQ to 150% of analyte concentrations (40%, 60%, 80%, 100%,
120% and 150%). The calibration curve was produced by plotting the average of triplicate of p-Toluenesulfonicacid
injections against the concentrations expressed in percentage. The slope, intercept and correlation coefficient values
were derived from linear least-square regression analysis and the data were presented in Table 2. It reveals that good
correlation existed between the peak areas concentration of p-Toluenesulfonicacid. Repeatability was checked by
calculating the relative standard deviation (%RSD) of six determinations by injecting six freshly prepared solutions
containing and 1.5 ppm of p-Toluenesulfonicacid on the same day. The low %RSD values confirm the good precision
of the developed method. Table 3. |
 |
 |
CONCLUSION
|
The present development study is based on validation of a highly sensitive, specific, reproducible and high-throughput
LC-MS/MS method to quantification of p-Toluenesulfonicacid in APIs. It has been established that it is highly
sensitive with a limit of detection (LOD) of 0.5 ppm Trace level ammonium acetate is added to the mobile phase to
enhance ionization and detection. Selected sample solvents were assessed for the effect on standard stability with and
without presence of API. As a systematic approach, it is very important to utilize the comprehensive chromatographic
knowledge gained throughout the lifecycle of the development of a drug candidate based on continuous understanding
of the API manufacturing process. The method which is able to quantify them at ppm level is developed and validated.
We can conclude that the developed method could be very useful for monitoring of p-Toluenesulfonicacid in acyclovir
in its pure and tablet form. |
References
|
- Allen RA, Schwartz RA, Rosea P. PityriasisRosea. 2009. http://www.emedicine.com/DERM/topic335.htm
- Attili VSS, Singh VP, Sundar S, Gulati AK, Varma DK, Rai M. Relationship between skindiseases and CD4 cell count in a hospital based cohort of HIV infected adults in NorthIndia. Journal, Indian Academy of Clinical Medicine (JIACM).2008;9(1):20-5.
- Bhandary PG, Kamath NK, Pai GS, Rao G. Cutaneous manifestations of HIV infection.Indian J DermatolVenereolLeprol. 1997;63:35-7.
- Boonchai W, Laohasrisakul R, Manonukul J, Kulthanan K. Pruritic papular eruption in HIVseropositive patients: a cutaneous marker for immunosuppression. InternationalJournal of Dermatology. 1997; 38(5):348-350.
- Bravo IM, Correnti M, Escalona L, Perrone M, Brito A, Tovar V, et al. Prevalence of orallesions in HIV patients related to CD4 cell count and viral load in a Venezuelanpopulation. Med Oral Patol Oral Cir Bucal. 2006;11:E1-5.
- Coopman SA, Johnson RA, Platt R, Stern R. Cutaneous disease and drug reactions in HIVInfection. N Engl J Med 1993;328(23):1670-4.
- Hengge UR, Franz B, Goos M. Decline of infectious skin manifestations in the era of highlyactive antiretroviral therapy, AIDS. 2000;14(8):1069.
- HIV Estimates in India for the year 2006. NACO document;http://www.naco.nic.in/indianscene/esthiv.htm.
- Kumarasamy N, Solomon S, Madhivanan P, Ravikumar B, Thyagrajan SP, Yesudian P.Dermatological manifestations among human immunodeficiency virus patients inSouth India. Int J Dermatol. 2000;39:192-5.
- Maurer T, Rodrigues LKE, Ameli N, Phanuphak N, Gange SJ, DeHovitz J, French AL,Glesby M, Jordan C, Khalsa A, Hessol NA. The Effect of Highly ActiveAntiretroviral Therapy on Dermatologic Disease in a Longitudinal Study of HIV Type1-Infected Women. Clinical Infectious Diseases. 2004;38(4):579-584.
- Mbuagbaw J, Eyong I, Alemnji G, Mpoudi N, Same-Ekobo A. Patterns of skinmanifestations and their relationships with CD4 counts among HIV/AIDS patients inCameroon. Int J Dermatol. 2006;45:280-4.
- Munoz-Perez MA, Rodriguez-Pichardo A, Camacho F, Colmenero MA. Dermatologicalfindings correlated with CD4 lymphocyte counts in a prospective 3 year study of 1161patients with human immunodeficiency virus disease predominantly acquired throughintravenous drug abuse. British Journal of Dermatology. 1998;139(1):33-39.
- Nair SP, Moorty KP, Suprakasan S. Clinico-epidemiological study of HIV patients inTrivandrum. Indian J DermatolVenerolLeprol. 2003;69:100-3.
- Samet JH, Muz P, Cabral P, Jhamb K, Suwanchinda A, Freedberg KA. Dermatologicmanifestations in HIV-infected patients: a primary care perspective. Mayo Clin Proc.1999;74(7):658-60.
- Sen S, Halder S, Mandal S, Pal PP, Halder A, Bhaumik P. Clinico-epidemiological profile ofcutaneous manifestations among human immunodeficiency virus positive patients inthe sub-Himalayan region. Indian J DermatolVenereolLeprol. 2009;75:403-5.
- Sengupta D, Rewari BB, Mishra SN, Joshi PL, PrasadaRao JVR. Spectrum of opportunisticinfections in AIDS: Trends from India. JIACM. 2000;4:99-103.
- Shobhana A, Guha SK, Neogi DK. Mucocutaneous manifestations of HIV infection. Indian JDermatolVenereolLeprol. 2004;70:82-86.
- Singh H, Singh P, Tiwari P, Dey V, Dulhani N, Singh A. Dermatological manifestations inHIV-infected patients at a tertiary care hospital in a tribal (Bastar) region ofChhattisgarh, India. Indian J Dermatol. 2009;54(4):338-41.
- Sud N, Shanker V, Sharma A, Sharma NL, Gupta M. Mucocutaneous manifestations in 150HIV infected Indian patients and their relationship with CD4 lymphocyte count.International Journal of STD and AIDS. 2009;20:771-774.
- Rajagopalan B, Jacob M, George S. Skin lesions in HIV positive and negative patients inSouth India. Int J Dermatol. 1996;35(7):489-92.
- Lakshmi SJ, Raghurama GR, Ramalakshmi, Satyashree, Rao KA, Prasad PG, Kumar YHK.Pruritic papular eruptions of HIV: A clinicopathologic and therapeutic study. Indian JDermatolVenereolLeprol. 2008;74(5):501-3.
- Orlovic D, Smego RA. Hypercoagulability Due to Protein S Deficiency in HIV-SeropositivePatients. International Journal of Collaborative Research on Internal Medicine &Public Health (IJCRIMPH), 2009;1(6&7):187-193.
- Rajendran PM, Dolev JC, Heaphy MR, Maurer T. Eosinophilic Folliculitis: before and afterthe introduction of antiretroviral therapy. Arch Dermatol. 2005;141(10):1227-1231.
- UNAIDS update of the global HIV/AIDS Epidemic. December 2007.
|