ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
|
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
In the present work, the effect of electrochemical bath parameter like corrosion rate on physical and mechanical properties of Nickel based SiC composite coating on Mild Steel substrate was studied. Ni-SiC nano-composite coatings were prepared on a MS substrate by electro-co-deposition process using a sulphamate bath. Corrosion rate, by using a salt spray test, was measured at different experimental conditions and the results were tabulated. The experimental results showed that the corrosion rate was lowest at SiC loading of 4 gm/l, 3 A/dm2 of Current density and 45oC of bath temperature
Keywords |
| Keywords; electro-co-deposition, corrosion, electrochemical bath, composite coating. |
INTRODUCTION |
| Composite coating is a highly advanced method in material science, as it produces new materials at room temperature and appropriately it is referred to as cold fusion. The first investigation started in the early sixties and soon after this, new industrial applications for electro-co-deposited composites were found. Interest in the technique grew and a better understanding of the process was obtained. There are other methods of producing composite materials with a metallic matrix. The purpose of composite coating is to give various functional properties, such as wear resistance, self-lubrication, corrosion or oxidation resistance etc., to the plated surface. The most widely used methods are powder metallurgy, and internal oxidation [1-2]. A major disadvantage of these methods is that they need to be performed at high temperatures. Furthermore, these methods often do not allow the production of thin foils. Because electroplating is a very suitable method to produce metal coatings, electrochemical co-deposition is a good alternative in the field of composite coatings. |
EXPERIMENTAL STUDY |
| Specimen is made up of mild steel rectangular plate of 35mm X 15mm X 2mm shown in fig .1 was used as a substrate. It is subjected to surface buffing for smoothing the surface of substrate. Buffed specimens were cleaned up with soap, water and ultrasonically degreased with acetone and then rinsed with a 5% sulfuric acid solution at room temperature for 5 minutes. Finally, these specimens were rinsed in de-ionized water for 5 minutes. Before coating, the specimen’s initial weight is taken on the AFCOSET electronic balance machine which as shown in the Fig 2. |
 |
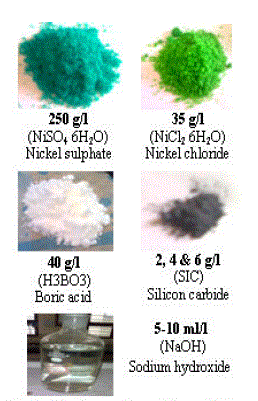
| The chemical reagents given in Fig. 3 and distilled water were used to prepare the electro- co-deposition solution. The reinforcement SiC size of 2-3ïÃÂÃÂm was used for electro- co-deposition. After the electrolytic solution is obtained and the 400ml is transferred in a small beaker and the SiC powder was added to the bath at a different concentrations of 2, 4, and 6g/l. The blend was sonicated in an ultrasonic bath machine for one hour, such that all the reinforcement SiC particles were sufficiently wetted and remained suspended uniformly in the solution. After sonication, the suspension was transferred to electrolytic solution and diluted to the required concentration. The pH values were checked by pH instrument and pH value of 3.5 is set by adding sodium-hydroxide (NaOH) solution. |
 |
| Electroplating is carried out with the help of electro co-deposition coating equipment as shown in Fig. 5. Specimen pin is covered with 3M masking tape on outer surface except the buffed area where the coating is done. Similarly 9 specimen cylindrical pins are masked and made into a single bunch. Pure nickel rod acts as anode and mild steel specimen bunch as cathode onto which desired material is coated. Both the anode and cathode are dipped into the composite electro-co-deposition solution. The distance between the electrodes is maintained at 9cm for all experiments. The electrolyte is continuously stirred with the help of magnetic stirrer at 250 rpm and heated to required temperature. Both current density (2, 3 & 4 A/dm2) and bath temperature (45, 55 & 65ºC) are set to desired values. |
| The electroplating was done for 60 min. The solution was continuously stirred at regular intervals to avoid settling down of SiC. The bunch after coating were removed and was washed with water for 10 min in order to remove loosely adsorbed reinforcement particle from the coating surface. Once the coating process completed, the specimens were removed from the bunch and the weight of individual specimen was noted down. This would be the weight of specimen after coating or before the wear test. |
| The specimen after salt spray test is as shown in the Fig.5. The most frequently applied accelerated corrosion test is the ASTM B 117 neutral salt spray test. Coated steel specimens were exposed to a fine-fog salt solution at an elevated temperature (35°C) and the percentage of the specimen surface covered in red rust was measured as a function of exposure time. |
 |
| 60 Liter of 5% NaCl (Sodium chloride) solution was filled into reservoir. The temperature of the chamber was maintained at 35°C. The salt solution fell on the specimens at a rate of 1.0 to 2.0 ml/80cm²/hour. The fogging of the salt solution was done at the specified rate. The samples were placed at a 15-30 degree angle from vertical. This orientation allowed the condensation to run down the specimens and minimized condensation pooling. Fig 2 shows the Ni- SiC coating after corrosion. |
 |
 |
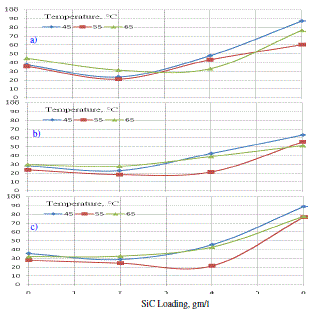
| The corrosion test by the weight loss method Fig 6 (a-c) examined in an aggressive environment (NaCl 3.5%) and ambient temperature showed that the corrosion rate initially decreased with increasing SiC loading and then it increased with SiC loading due to irregular exposure surface area [11]. This lead to more exposure area for corrosion. |
| Experiments showed that the loading composites were found to be less corrosion resistant than pure nickel and lower loading composites and as the SiC content increased after 4 g/l in the deposit, its corrosion resistance decreased. Such behaviors was already been reported in the literature [11]. It was explained that the initial corrosion started around the particles i.e., the metal particle boundary as the anodic region. |
 |
| Since the number of particles in the bulk of the coating was enormous, a continuous distribution of the corrosion current occurred on the anodic area. The inclusions could act as favorable sites for corrosion and acceleration. The particles could become dislodged during dissolution, exposing more area to corrosion, and was confirmed by the corrosion potential showing a positive shift. |
| For most of the cases, the Nyquist plots were semicircle indicating the activation control of the corrosion reaction. |
| The result indicates that the addition of smaller SiC was beneficial to corrosion resistance of the composite coatings. The effects of SiC on corrosion resistance of the composite coating could include two opposite aspects: On one hand, the potential of carbon was more positive than that of nickel in solutions and the potential difference between SiC and surrounding nickel metals could accelerate corrosion of nickel matrix. In addition, the increased conductivity and increased boundary areas between SiC and nickel matrix were also detrimental to corrosion resistance of the coating. On the other hand, the potential difference between SiC and nickel could also promote passivation of nickel and lead to the increase of corrosion resistance. Under our experimental condition, the latter effect played a more important role, which was confirmed by the increase of coating resistance Rp of the composite coating. It was noted that the coating deposited under the current density of 2 A/cm2 showed higher Rp value than the coating obtained at 3 A/cm2, This phenomenon indicated that there were some other factors affecting corrosion behavior of the composited coating in addition to the SiC. A possible explanation was the effect of grain size. During the electro-co-deposition of nickel, the grain sizes of nickel deposit decreased with the increase of current density [12], which was beneficial to corrosion resistance of the coatings. |
 |
| Fig.7 shows the SEM images of the corrosion morphology of Ni-SiC coating obtained after weight-loss test in 3.5 wt. % NaCl solution for 100 hours for the 27 samples, the surface indicates serious corrosion taking place on it. The corrosion surface of Ni-coating contained some pits, few cracks and flakes indicating the degree of corrosion was little higher in Ni-SiC 2 g/l composites coatings. Ni-SiC 4 g/l composites showed that shallow, few and small pits. The result indicated that the Ni-SiC composite coating had best resistance to corrosion, close to result obtained by weight loss method and electrochemical measurement. |
| The SEM images of Ni-SiC composite-coated samples exhibited the corrosion products on the entire surface, but compositecoated samples showed negligible amount of corrosion products. These results inferred that the composite coated sample retained its original surface even after 15 days of immersion in NaCl and possessed higher corrosion resistance property. But higher bath Ni-SiC 6 g/l loading composite coatings showed more porosity and corrosion. |
CONCLUSIONS |
| Corrosion rate was smaller in composite coated sample. |
| SEM images indicated the negligible corrosion at the composite coating surface. |
| The corrosion tests realized using the lost weight method showed that the corrosion rate was decreased with Ni-SiC composite coating. |
| The result obtained indicated that, the Ni-SiC composite coating had best resistance to corrosion at a SiC loading of 4 g/l. |
FUTURE DIRECTIONS |
| Cadmium plating is used for corrosion inhibition on diverse items such as aircraft components, locks and fasteners. However, cadmium electroplating is coming under increasing pressure due to both environmental and work safety issues. The use of cadmium and hexavalent chromium on vehicles and electrical equipment is restricted by the regulations of European ELV, WEEE and RoHS, except for certain defense related applications. |
| There is a scope to establish Ni-SiC coating as a potential replacement for cadmium coatings for the above reasons. |
References |
|