ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Shantha Margaret1, Uma Maheswari1, Ambethkar1, Vasudevan2, Sivanandhan2, Selvaraj3*
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
The present study was undertaken with a view to develop an efficient protocol for in vitro multiple shoot formation and subsequent root induction considering various cultural aspects using nodal explants of Cucumis anguria L. derived from 20 day - old in vitro seedlings. High frequency of multiple shoot regeneration was achieved on MS medium containing BAP (1mg/l), NAA (0.2mg/l) and L - glutamine (20mg/l). The elongation of shoots was obtained on the MS medium fortified with GA3 (0.5mg/l). Elongated shoots were rooted in MS medium supplemented with IBA (0.6mg/l). Rooted plants were hardened and acclimatized. Seventy percent plantlets survived and grew successfully. This protocol yielded an average of 6.2 shoots per explant in culture duration of 120 - 140 days.
Keywords |
| In vitro, MS medium, nodal explants, direct regeneration, Cucumis anguria. |
I. INTRODUCTION |
| The West Indian Gherkin (Cucumis anguria L.) is an important horticultural crop belonging to the family Cucurbitaceae. The fruits are oval in shape with highly warted skin and long spines. In India, it has been grown for export and local medicinal use. It is mainly cultivated for fruits which are used for salads and pickles (Grubben and Denton, 2004). The roots, fruits and seeds are used in the traditional medicine to treat jaundice, stone formation and stomach ailments (Schultes, 1990). The West Indian Gherkin is resistant to Cucumber Green Mottle Virus (CGMV) (Kroon et al., 1979), Zucchini Lethal Chlorosis Virus (ZLCV) (Giampan et al., 2007), Fusarium Wilt (Fusarium oxysporum, Thomas and More, 1990) and Powdery Mildew (Lebeda, 1984). However, this plant is seriously affected by Leaf Spot disease which causes more than 75% crop damage (Kudela and Lebeda, 1977 ). Conventional crosses and transfer of desirable traits, especially disease resistance from wild species have not been accessible in Cucumis (Esquinas - Alcazar and Gulick, 1983). The intention of biotechnology is to improve the crops by means that are impossible by conventional breeding (Gaba et al., 2004 ). |
| Many important crop plants are propagated vegetatively and grown as clones but West Indian Gherkin is usually propagated by sexual seeds. However, for the development of transgenic plants, in vitro regeneration is required at least for the initial establishment of the plant irrespective of the mode of propagation (Mohiuddin et al., 1997 ). Both direct and indirect organogenesis have been reported in cucumber cultivars but no reports are available yet in Cucumis anguria L. Direct shoot regeneration is less time consuming with less abnormality observed in the regenerants. |
II. LITERATURE SURVEY |
| Direct organogenesis has been reported for many cucurbits from various explants viz., cotyledons (Misra and Bhatnagar, 1995 ), hypocotyls (Mohiuddin et al., 1997), cotyledonary node (Ambethkar et al., 2012). The aim of present study was to establish an effective and reproducible protocol for in vitro regeneration of multiple shoots from nodal explants of C. anguria by direct regeneration. |
II.MATERIALS AND METHODS |
| Source of seeds: |
| Seeds of West Indian Gherkin (Cucumis anguria L.) variety AJAY F1 were procured from Nunhems India Pvt. ltd., Andhra Pradesh, India - 501403. |
| Sterilization of Seeds: |
| Mature seeds were used as explants source. The seeds were surface sterilized by washing with 3-5 drops of ‘Teepol’ brand (commercial bleach solution, 0.6% sodium hypochlorite, Rickitt and Benckiser Ltd., Kolkatta, India) for 5 minutes and rinsed with distilled water five times to remove the soap solution. To reduce the fungal contamination, the soaked seeds were treated with 70% ethyl alcohol for 30 seconds and then rinsed with sterile distilled water for three to five times. Then the seeds were surface sterilized with 0.1% (w/v) mercuric chloride solution for three minutes. Finally the seeds were rinsed with sterile distilled water for five times and left to air dry. The explants were treated with the fungicide (bavistin) for 15 minutes and rinsed again with distilled water for 3-5 times. |
| Explants Source: |
| The surface sterilized seeds were aseptically inoculated on MS basal medium (Murashige and Skoog, 1962) containing in culture tubes (25mm x 150mm; Borosil, India) for germination. The inoculated seeds were kept in dark for 2 days to render uniform germination and then in light (18/6 photoperiod) with the intensity of 50μ mol-2ms-1 for subsequent days provided by cool white light. Nodal explants (1.0 cm) were excised from 20 day old in vitro raised seedlings and used as explants. |
| Shoot induction and proliferation: |
| The nodal explants were placed vertically with the proximal region facing up in culture tubes (1 nodal explant per tube) containing 20ml of MS medium 3% (w/v) sucrose, 0.8% agar fortified with different concentrations of BAP (0.5 - 2.5 mg/l) Kn (0.5 - 2.5mg/l) and NAA (0.2 - 1.2mg/l). The effect of L - glutamine of various concentrations (5 - 25 mg/l) in the medium was tested for shoot initiation and proliferation. The pH of medium was adjusted to 5.8 prior to autoclaving for 20 minutes at 1.05 kg cm-2 at 1210 C. The cultures were maintained at 25 ± 20C up to 16 hr photoperiod with light intensity of 30 μmolm-2 s-1 provided by cool white fluorescent light (Philips India). The shoots produced from the initial cultures were sub cultured for five times with 25 days interval. |
| Shoot elongation, rooting and acclimatization: |
| The proliferated multiple shoots with an average length of 1 - 2 cm were carefully excised from node and were transferred to shoot elongation medium containing BAP (1.0 mg/l) and different concentrations of GA3 (0.2 - 1mg/l). The cultures were maintained at 25 ± 20C up to 16 hr photoperiod. After 3 weeks, shoots longer than 4 cm were selected and transferred to rooting medium. Elongated shoots (3-6 cm in height) were transferred to MS medium containing BAP, along with different concentrations of IBA (0.2 - 1mg/l) for rooting. The cultures were maintained as described above. After 2 weeks of culture in the root induction medium, the rooted plants were washed in running tap water to remove agar from root surfaces and transferred to paper cups containing sterile soil, sand and vermiculite (1:1:1 v/v/v) and were placed in shade house. The plants were watered daily. After 3 - 4 weeks, the acclimatized plants were transplanted to earthen pots and were grown in garden. |
| Statistical Analysis: |
| Each treatment factor consisted of 20 replicates and the experiment was repeated 3 times. Data on multiple shoot regeneration, elongation and rooting were statistically analyzed by using Duncans Multiple Range Test (DMRT). Significance was determined at P < 0.05 by using the software SPSS, Windows 7 Operating System. |
III. RESULTS AND DISCUSSION |
| Multiple shoot bud induction and proliferation |
| Multiple shoot buds originated from nodal explants in MS medium supplemented with different concentrations of BAP (0.5-2.5mg/l), KN (0.5-2.5 mg/l) and NAA (0.2-1.2mg/l). The effect of BAP (1.0 mg/l) with varying concentrations of Kn and NAA were tried. The highest percentage of multiple shoot induction was observed on MS medium supplemented with 1.0 mg/l BAP + 0.2 mg/l NAA (Table-1, Fig-1 b & c) with the maximum mean number of shoots/explant was recorded. Presence of BAP either alone or in combination with other cytokinins proved essential for direct as well as indirect shoot regeneration in cucurbits (Chaturvedi and Bhatnagar, 2001 ). Earlier reports recorded the formation of multiple shoots in cucurbits in in vitro by using different explants with cytokinins alone (BAP/Kn) (Mahzabin et al., 2008 ) or combination of cytokinins (BAP/Kn) with auxins (NAA, IAA and IBA). BAP (88.1%) and Kn (64.4%) induced multiple shoots from shoot tip explants of pumpkin with the production of 14 and 6 shoots/explants respectively (Mahzabin et al., 2008 ). In pumpkin the nodal explants produced considerable percentage (85% and 90%) of multiple shoots (Haque et al., 2008 ). Ahmad and Anis (2005 ) observed 96% of shoot regeneration from nodal explants of Cucumis sativus. Devendra et al. (2008) observed the maximum percentage of (76.6 %) multiple shoot regeneration from BAP and NAA combination in shoot tip explants of T. cucumerina. Multiple shoots were induced from shoot tip explants of two genotypes of pointed gourd by BAP alone and in combination of NAA (Malek, 2007). Khatun et al. (2010) reported that the BAP and NAA resulted in maximum shoot induction from nodal explants of water melon. In melon cotyledonary node explants, the combined effect of BAP and NAA resulted in higher frequency of multiple shoots regeneration (Zhang et al., 2011). Nodal explants of pointed gourd produced multiple shoots in BAP, Kn & NAA combination (Komal, 2011). L - glutamine in nodal explants induced multiple shoots due to its promotive effect. The released nitrogen sources from L – glutamine provides a readily available source of nitrogen, the implication being that the formation of necessary carbon skeleton or the reduction of nitrate to ammonia is a limiting factor in the cells (Bayley et al., 1972). Maximum multiple shoot enhancement was observed in Cucumis melo due to the addition of glutamine (Muruganantham et al., 2002). Selvaraj (2002) reported that L - glutamine (137μm) improved the shoot production in the shoot tip culture of Cucumis sativus L. Vasudevan et al. (2004) reported in cucumber that the supply of L - glutamine produced highest number of shoots/explant. The sub cultures of shoots were carried out in MS medium containing BAP (1.0 mg/l) NAA (0.2 mg/l) & L - glutamine (20 mg/l) at 25 days of interval for 3 times. There was a marginal increase in the number of shoots per explant was noticed in the first 2 subcultures. After 2 subcultures there was no further improvement in the number of shoots. Nodal explant produced an average of 7 shoots after the end of three subcultures. |
 |
| Fig. 1. Direct regeneration of multiple shoots from nodal explants derived from 20 day-old in vitro seedlings of C. anguria L. a) Nodal Explant, b) Shoot induction in MS medium with BAP (1 mg/l) + NAA (0.2 mg/l) + L - glutamine (20 mg/l) c and d) Shoot proliferation in MS medium with BAP (1 mg/l) + L – glutamine e) Shoot elongation in MS medium with BAP (1 mg/l) + GA3 (0.5 mg/l). f) Rooting in MS medium with BAP (1 mg/l) + IBA (0.6 mg/l) g) acclimatized plant. |
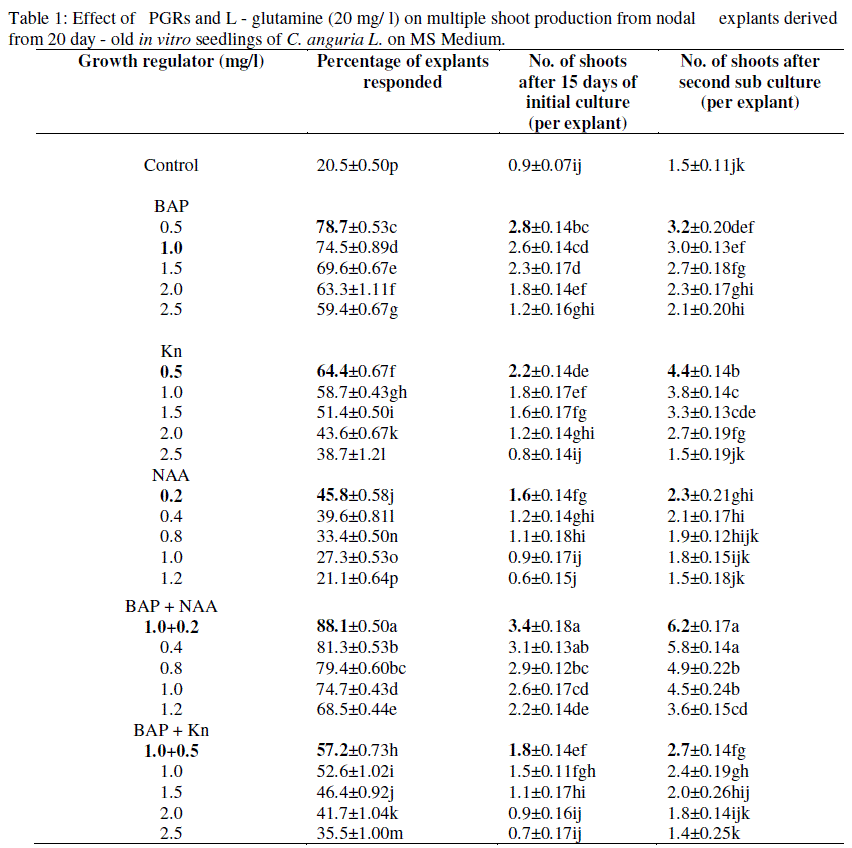 |
| Control: without growth regulator. Data presented as means ± SE from 20 explants for each treatment and repeated three times. Means followed by same letters within a column are not significantly different according to Duncan’s Multiple Range Test (DMRT) at P ≤ 0.05 level. |
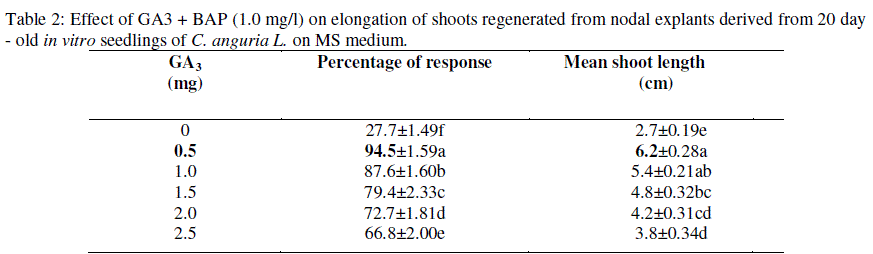 |
| Control: without growth regulator. Data presented as means ± SE from 20 explants for each treatment and repeated three times. Means followed by same letters within a column are not significantly different according to Duncan’s Multiple Range Test (DMRT) at P ≤ 0.05 level. |
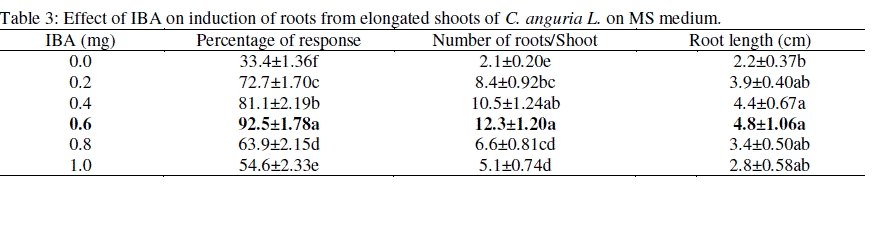 |
| Control: without growth regulator. Data presented as means ± SE from 20 explants for each treatment and repeated three times. Means followed by same letters within a column are not significantly different according to Duncan’s Multiple Range Test (DMRT) at P ≤ 0.05 level. |
| Shoot elongation, rooting and acclimatization: |
| Emerging shoots (2 - 3 cm long) were transferred to shoot elongation medium (SEM) containing either GA3 alone or in combination with BAP (1.0 mg/l) (Table-2). Shoots grown in the medium with GA3 alone were shown to be slender, weak and with narrow leaves (data not shown). On the other hand, MS medium with BAP and GA3 combination produced healthy and normal shoots. Hence in the present study different concentrations of GA3 (0.2 - 1mg/l) were tested along with BAP (1.0 mg/l). Maximum shoot elongation (6.2 cm) and percentage of response (94%) were recorded in GA3 (0.5 mg/l) and BAP (1.0 mg/l) (Fig-1 d). The elongated shoots appeared normal and healthy. NAA responded poorly with the production of poor, weak and slender roots when compared with IBA (0.6 mg/l) (Table-3; Fig-1 e). The present study revealed that IBA was effective on root induction ability from the elongated shoots. Agarwal and Kamal, (2004) obtained similar results in Momordica charantia and Haque et al. (2007) in Mormordica dioica. The rooted plants were transferred to small pots for hardening. After 3 - 4 weeks of hardening the plantlets were subsequently transferred to the field for acclimatization. Finally 70% plants survived. Similar kinds of results were obtained in acclimatization by Compton et al. (2001) for C. hystrix. |
IV. CONCLUSION |
| In the present study maximum number of shoots (6 - 8) was regenerated directly on nodal explants derived from 20 days old in vitro grown seedlings. Combination of BAP (1.0 mg /l), NAA (0.2 mg/l) and L - glutamine (20 mg/l) favoured multiple shoot bud induction and proliferation. Elongation of shoots was achieved in GA3 (0.5 mg/l + BAP 1.0 mg/l) and elongated shoots were rooted in IBA (0.6 mg/l). This simple protocol is reliable and may be useful for propagation of Cucumis anguria in a shorter period and can also be utilized for genetic transformation studies. |
References |
|