ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
| Govardhan Naik A1, Vengaiah V2, Lalithamma A3 Changamma C4 |
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
The oral administration of Betel leaf stalk extract in male albino rats shows no influence on structural organization of liver. Reduction in liver proteins but elevation in serum proteins might be due to the mobilization towards blood. Reduction in liver and serum carbohydrates indicates hypoglycemia & some hepatic cell proliferation by administration. Due to the administration of leaf stalk extract more glucose is metabolized in to gluconate in liver leads to lowered serum glucose levels. There was no significant changes occur in liver glycogen over treatment represents anti-hyperglycemic activity of extract. Lactate which is an end product of the glycolytic pathway is an index of the relative aerobic or anaerobic nature of the tissue understudy. The enhanced pyruvic acid was due to higher glycolysis and more lactic acid was converted in to pyruvic acid.
Keywords |
| Betel leaf stalk extract, liver, serum, hypoglycemia, lactic acid |
INTRODUCTION |
| Several plant products inhibit male and female fertility and may be developed in to contraceptives. Even though, many indigenous plants have been shown to prevent the birth, only few plants have so far been investigated for anti-fertility activity. Several plant species have been described as antifertility agents [1]. Oral feeding of male rats with the ethanolic leaf extract of Colebrookia oppositifolia at dose levels of 100 and 200 mg/kg for 8-10 weeks respectively did not cause body weight loss, while the weights of testes and epididymis were significantly decreased. Also reported that depression of spermatogenesis, decrease in the primary and secondary spermatocytes, sperm count, motility and fertility Gupta et al. [2] Reports on decrease in the number of primary spermatocytes, interference and arrest of spermatogenesis, reduction in male fertility by extract of Tinospora cordifolia [3], Eurycoma longifolia Wahab et al. [4], Ruta graveolons Bazrafkan et al. [5], Mondia whitei Watcho et al. [6] have been documented. The betel leaf (Tambula patra brint) is extensively cultivated in warm moist parts of India for its leaves; the antifertility properties of the betel plant were studied in both male and female rats. It was suggested that the contraceptive effect of the extract of leaf stalk of piper betel Linn is mainly on the maturation process of spermatozoa in epididymis without influencing hysteric hormonal profiles Tewari et al. [7] .To find out the toxicity potential of piper betel leaf stalk extract, the studies on liver and serum profiles are neccesary. Hence the present study was undertaken. |
II. MATERIAL AND METHODS |
| In the present study healthy adult (3 months old, weight 160±10g) male wistar strain albino rats were used. The rats were purchased from Sri Raghavendra Enterprises, Bangalore, India. The male albino rats were taken and divided in to two groups, each group contains 6 rats. First group are control rats administered with 1 ml of distilled water. Second group are experimental, administered with betel leaf stalk extract, at the dose rate of 150 mg/Kg body weight/day through oral gavages method Dehgan et al. [8] for 15 days. Animals were housed in a clean polypropylene cage under hygienic conditions in well ventilated clean air conditioned room, with photoperiod of 12 hours light and 12 hours dark cycle, at 25 ± 2ºC with a relative humidity of 50 ± 5%. The rats were fed with standard laboratory feed (Hindustan Lever Ltd, Mumbai) and water ad libitum. Twenty four hours after the last dose, the animals were autopsied. The tissues like liver were isolated, chilled immediately and used for biochemical analysis. Blood was collected, separated serum and used for biochemical analysis. Dry matter and water content were analyzed gravimetrically. The total proteins Lowry et al. [9], total carbohydrates Carrol et al. [10], total lipids Folch et al. [11], glucose Mendal et al. [12], glycogen [13], lactic acid [14&15] and pyruvic acid [16] were estimated biochemically both in control and experimental rat tissues. |
III. RESULTS AND DISCUSSION |
| The liver is the major metabolic organ. In the present study there was no significant changes in dry matter and water content, represents that the betel leaf stalk extract does not shows any influence on structural organization of liver. There was significant reduction in hepatic proteins and elevation in serum proteins. It might be due to the mobilization towards blood [17], Hasimbasha et al. [18]. Reduction in liver and serum carbohydrates indicates hypoglycemia & some hepatic cell proliferation [19] by administration. Total lipids decreased in liver due to mobilization towards blood circulation [20&21]. The glucose levels were significantly reduced in liver and serum (-49.59 P<0.001, -7.36 p<0.001) over control. Liver has a major role in energy storage in both humans and animals, males might be predicted to more rapidly mobilize hepatic carbohydrates as compared to females [22]. Liver, the chief site for intense metabolism and excretion, it regulates homeostasis of the body Ahsan et al. [23]. Liver is the primary organ for glucose metabolism. Apart from expressing the enzymes involved in glucose metabolism and regulation, liver possesses numerous enzymes involved in detoxification and toxicity enhancement [24]. Glucose is an essential nutrient for the human body. It is the major energy source for many cells, which depend on the blood stream for a steady supply. Blood glucose levels, therefore, are carefully maintained. The liver plays a central role in this process by balancing the uptake and storage of glucose via glycogenesis and the release of glucose via glycogenolysis and gluconeogenesis. The several substrate cycles in the major metabolic pathways of the liver play key roles in the regulation of glucose production Nordile et al. [25]. In the present findings there was 50% reduction in hepatic glucose and 7% reduction in serum glucose levels over control. It is due to the administration of leaf stalk extract more glucose is metabolized in to gluconate in liver which leads to lowered serum glucose levels. There was no significant changes occur in liver glycogen over treatment. Liver glycogen level may be considered as the best marker for assessing anti-hyperglycemic activity of any drug Grover et al. [26]. Liver is the major tissue to convert glucose, fatty acids and amino acids to acetyl-CoA [21]. Thus it also indicating nontoxic nature of plant material administered. In liver the lactic acid was reduced significantly. Lactate which is an end product of the glycolytic pathway is an index of the relative aerobic or anaerobic nature of the tissue understudy. It is converted in to pyruvic acid before it can be metabolized and the conversion of lactate into pyruvic acid is depending upon NAD concentrations and thereby it showed enter into TCA cycle [27]. Decrease in lactate level might be due to increased conversion to pyruvate under aerobic conditions. The reduction in lactate content might be attributed to defect in catabolising glycogen in vitro Anil Kumar et al. [28].The depletion could also be attributed to gluconeogensis or utilization [29] or the possibility of glycogenolysis Neff et al. [30], [28]. There was accumulation of pyruvic acid in liver and serum. The enhanced pyruvic acid was due to higher glycolysis and more lactic acid was converted in to pyruvic acid. This further supported the lactic acid deployment. Increased pyruvate level might be because of reduced uptake of the metabolite by the mitochondria membranes as reported by Annison et al. [31]. This might be because of the pyruvate contents slightly increase under the toxic conditions; pyruvate gets accumulated which indicates the extract interference with the normal metabolic activities [28]. Table: 1 Effect of Betel leaf stalk extract on Liver profiles in male albino rats. |
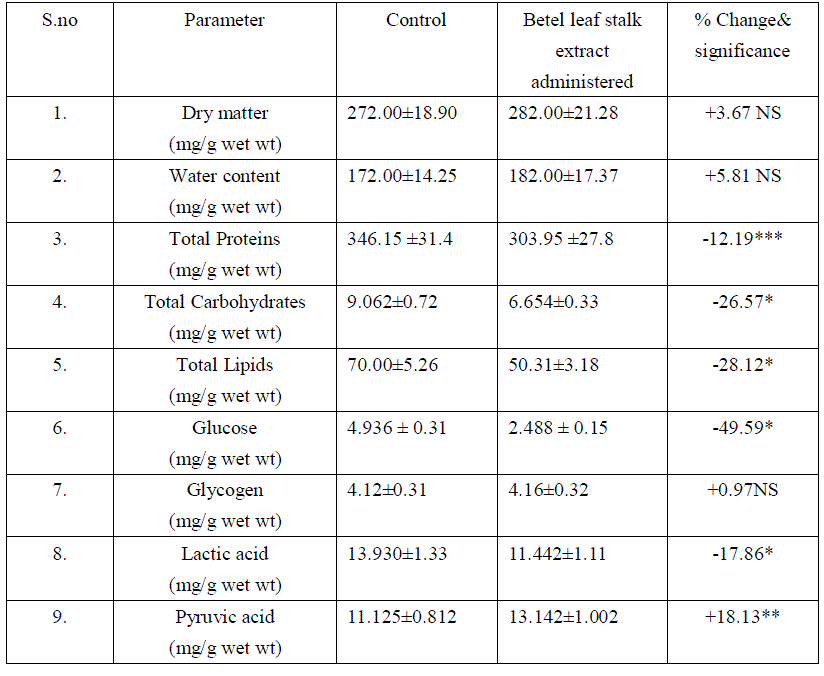 |
| Mean+ SD of six individual observations. + and – indicates percent increase and decrease respectively over control.*indicates P<0.001,** indicates P<0.01, *** P<0.05 indicates the level of significance. NS- non significant changes |
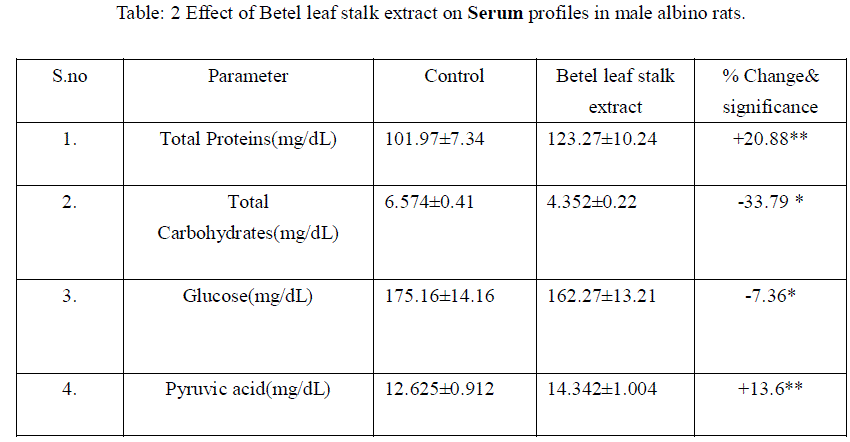 |
| Mean+ SD of six individual observations. + and – indicates percent increase and decrease respectively over control.*indicates P<0.001, ** indicates P<0.01 the level of significance. |
IV. CONCLUSIONS |
| The extract does not show any influence on structural organization of liver. Reduction in liver and serum carbohydrates indicates hypoglycemia & some hepatic cell proliferation by administration. No significant changes occur in liver glycogen over treatment may be considered as the best marker for assessing anti-hyperglycemic activity of extract. Enhanced pyruvate represents the extract interference with the normal metabolic activities. |
References |
|