ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Swaroopa Maralla1
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
From times unknown, ginger has become a subject of interest because of its beneficial effects on human health. The purpose of the present study was to investigate the effects of daily oral administration of ginger extract for 6 weeks on kidney functions in withdrawal rats to evaluate the ameliorating effects in alcohol induced-withdrawal rats. Rats (130-150gm) were divided into 4 groups; normal control rats, alcoholic control rats, ethanol withdrawal rats and ethanol withdrawal rats pretreated with ginger. Ginger extract was administered orally for 6 weeks to pre-treated rats, and they were compared with the normal and alcoholic groups, respectively. The treatment with ginger extract had significant effect on Plasma Electrolyte Profiles. Low plasma sodium level and increased plasma potassium levels were observed in ginger pretreated ethanol withdrawal group. The plasma creatinine, urea and uric acid levels were significantly reduced in this group compared to alcoholic control rats and ethanol withdrawal rats. It is concluded that the consumption of ginger produced a significant anti- nephrotoxic effect in ethanol withdrawal rats. In addition, ginger is showing properties of anti-hypertensive drugs and is capable of improving impaired kidney function in ethanol withdrawal rats.
Keywords |
| Ginger, Kidney Function, Alcohol, Plasma, Lipids, Withdrawal, Rats. |
I.INTRODUCTION |
| Long-term alcohol misuse is associated with water and salt retention, causing an expanded extracellular volume [1]. Impaired renal function, secondary to the long-term effects of alcohol misuse, also results in a metabolic acidosis, as well as other electrolyte disturbances such as hypomagnesaemia, hypophosphataemia and hypocalcaemia. Severe alcohol misuse predisposes to acute renal failure [1]. Rarely, bladder dysfunction occurs with alcohol misuse, possibly secondary to an alcohol-induced neuropathic bladder [2]. Urinary retention and abdominal distension can result. Excessive alcohol consumption can have profound negative effects on the kidneys and their function in maintaining the body’s fluid, electrolyte, and acid-base balance, leaving alcoholic people vulnerable to a host of kidney-related health problems that include decreased ability to excrete body wastes, inability to maintain body fluid and electrolyte balance and decreased synthesis of essential hormones [3]. |
| It is established that metabolic derangements are frequent in chronic alcoholics or even with acute intoxication and withdrawal[4]. The most common and potentially life-threatening abnormalities include hypokalemia, hypomagnesemia, hypophosphatemia, hypoglycemia, ketoacidosis and lactic acidosis. Severe alcohol withdrawal may have an important impact on fluid and electrolyte status. Almost all patients in acute withdrawal are hypovolemic as a result of diaphoresis, hyperthermia, vomiting, and tachypnea [5]. Hypokalemia is common due to renal and extrarenal losses, alterations in aldosterone levels, and changes in potassium distribution across the cell membrane [6,7]. Hypomagnesemia is common in patients with DT and may predispose to dysrhythmia and seizures [8]. Hypophosphatemia may occur due to malnutrition, may be symptomatic, and if severe, may contribute to cardiac failure and rhabdomyolysis ( [9], [10] ). |
| Plant derived products have been used for medicinal purposes for centuries. At present, it is estimated that about 80% of the world population relies on botanical preparations as medicines to meet their health needs [11]. Herbs and spices are generally considered safe and proved to be effective against many diseases ([12], [13]). Ginger has a long history of use in traditional medicine in the treatment of over a wide range of ailments including renal disorders. Ginger was proved more potent renoprotective agent in both acute and chronic renal failure (CRF) and the mechanisms underlying the effects of renal failure by ischemia–reperfusion ([14], [15]). The study of Ajith et al., 2007 [16] reaffirmed the protective effect of ginger against cisplatin-induced oxidative stress and acute renal failure in kidneys of mice. In addition, this study observed the effect of pre-treatment with ginger on the levels of serum creatinine and urea, and concluded that the administration of ethanol extract of ginger before and after cisplatin injection significantly lowered the elevated levels of serum creatinine and urea. |
| In light of these observations an attempt was made in this study to evaluate the nephroprotective ability of ginger extract during acute withdrawal from chronic alcohol ingestion in male wistar rats. |
II.MATERIALS AND METHODS |
| Ginger extract: The fresh rhizomes of Zingiber officinale were obtained from local market and identified by the herbarium staff of the Botany Department, SV University, India. Whole rhizome of ginger was thoroughly washed, sliced , grated and grind to fine paste . A weighed quantity (30gm) of the paste was subjected to continuous extraction in Soxhlet apparatus using double distilled water. The extract was evaporated under reduced pressure using rotary evaporator and then lyophilized until all the solvent has been removed to give an extract sample and stored at 4c for further studies. |
| Experimental animals: The study involved young (3–4 months old; 200 - 220g ) male albino rats of Wistar strain purchased from Sri Venkateswara Traders Pvt. Limited, Bangalore, maintained in the animal house of the department in polypropylene cages. The animals were allowed to habituate to the animal facilities for at least for two weeks adaptation period upon arrival and were maintained under standard conditions of humidity (50% relative humidity), room temperature (25 - 28ºC) and 12 h light/ dark cycle (6:00 A.M. to 6:00 P.M.). A standard rodent diet (M/s Hindustan Lever Ltd., Mumbai), and water were provided ad libitum. All experimental procedures were approved by the CPCSEA on Animal Care. |
| Experimental protocol: The experimental animals were divided into 4 groups; each group contained six animals: Control group G1 (normal without treatment), alcoholic group G2 (injected with 2 gm/ kg body wt ( p.o.) ethanol), alcoholic rats treated with ginger extract (200 mg/kg body weight) for 6 weeks G3(firstly, the rats were injected with ethanol along with ginger extract), alcoholic rats subjected to withdrawal G4(first, the rats were given Etoh for 6 weeks and then subjected to withdrawal from alcohol for 3 days after the last dose), withdrawal rats treated with ginger extract(200 mg/kg body weight) G5(first, the rats were given ginger for 6 weeks along with Etoh and then subjected to withdrawal from alcohol for 3 days after the last dose). Ginger extract was given orally (200 mg/kg body weight) to the rats through a gastric tube daily for 6 weeks. |
| Collection of blood samples: At the end of the 6 weeks of post-treatment, blood samples were collected by sacrificing the animals and the blood was collected in clean EDTA tubes, then plasma was separated by centrifugation and stored at-20°C for biochemical analysis. While the groups of rats pretreated with ginger were decapitated after 3 days of abstinence from chronic alcohol treatment and blood samples were collected as mentioned above. |
| Chemicals:All chemicals used were of analytical reagent grade. |
| Biochemical analysis: Creatinine and urea were determined by enzymatic method according to the method (Patton and Crouch, 1977) [17]. The flame photometry method of Vogel, 1960 [18] was used for the determination of sodium ion (Na+) and potassium ion (K+) concentration in serum. Inorganic phosphate (Pi) levels in serum was estimated according to Fiske and Subbarow, 1925 [19] method while serum Calcium and Magnesium were estimated using AAS according to the methods adapted from Thin and Thomson, 1967 [20]. |
| Statistical analysis: Data were statistically analyzed by one-way analysis of variance followed by Duncan's test (SPSS). Finally, significant difference (L.S.D) was used to test the difference among treatments. Results were considered statistically significant when (P < 0.05). |
III.RESULTS |
| Kidney Functions: As shown in Table 1, the alcohol produced significant increase in the levels of plasma creatinine, urea and uric acid when compared with normal group, while, administration of ginger extract to the alcoholic rats significantly reduced the levels of plasma creatinine, urea and uric acid when compared with the alcoholic group, but no significant changes were observed when compared with the normal rats. This indicates that, coadministration with ginger extract normalized the plasma creatinine, urea and uric acid. On the other hand, the pre-treatment of Etoh followed by induction of withdrawal decreased significantly the levels of plasma creatinine and uric acids were decreased significantly when compared with the alcoholic group. Furthermore, they are still significantly higher than normal rats. In contrast, nonsignificant increase was observed in plasma urea when compared with alcoholic group, but it was still significantly higher than normal rats as shown in Table 1. |
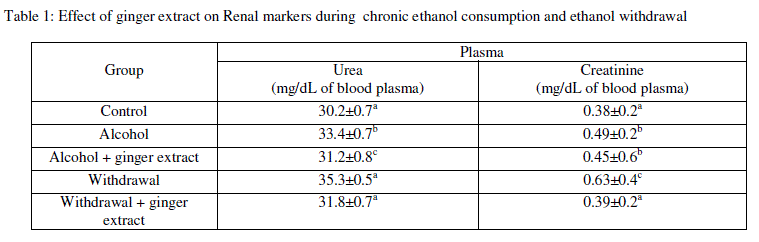 |
| Data are expressed as mean ± SE (n=6). The statistical test conducted was ANOVA followed by multiple two-tail t-test and data with different superscript (a,b,c) in a specific vertical column differed from each other significantly (p < 0.05). |
| Levels of serum electrolytes: Sodium and potassium levels were significantly increased in group II and group IV animals (the ethanol treated and withdrawal groups), compared to group I (control). But in group V (pretreatment with ethanol, withdrawal and coadministration of extract) significantly low levels of urea and creatinine were observed compared to group II and IV, and the values resettled to the levels of control group (Table 2). |
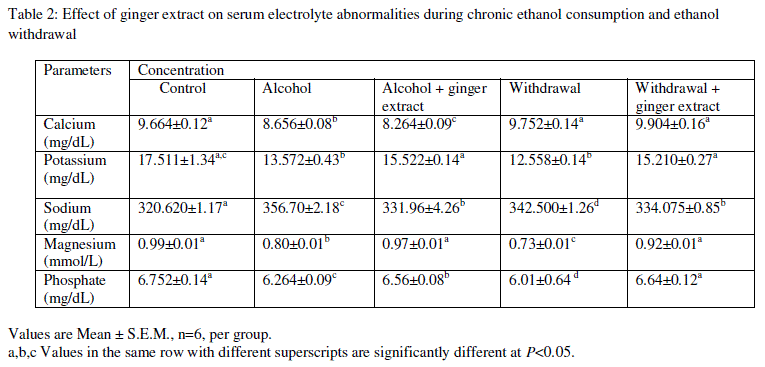 |
| Values are Mean ± S.E.M., n=6, per group. |
| a,b,c Values in the same row with different superscripts are significantly different at P<0.05. |
IV.DISCUSSION |
| The present study demonstrated that withdrawal induces the elevation of the plasma urea and creatinine in alcoholic rats, which are considered a significant marker of renal dysfunction [21]. In the present study the effect of ginger on the kidney functions was assessed by the determination the levels of plasma creatinine, urea and uric acid, and the study revealed that pre administration of ginger extract to the alcoholic rats reduced and normalized the levels of plasma creatinine, urea and uric acid. On the other hand, the pre-treatment with ginger before the induction of diabetes inhibited the higher increase of plasma creatinine and uric acid resulted from the induced-stress by EW but they did not normalized. Moreover, the study of Ajith et al [16] demonstrated that ethanol extract of ginger rendered significant protection against induced nephrotoxicity, which was evident from the lowered serum urea, and creatinine levels in the mice that were pretreated with ginger extract, and this study concluded that ginger extract significantly protected the elevation of serum creatinine and urea levels. Furthermore, the treatment of ginger extract could significantly prevent the depletion of antioxidant concentration and antioxidant enzymes activities in the kidneys. In addition, studies reported that the presence of polyphenols and flavonoids in ginger extract might be responsible for the antioxidant nephroprotective activities and the reduction of serum urea and creatinine levels [16]. |
| Withdrawal may also have an important impact on fluid and electrolyte status. Almost all patients in acute withdrawal are dehydrated as a result of diaphoresis, hyperthermia, vomiting, and tachypnea. Hypokalemia is common due to renal and extra-renal losses, alterations in aldosterone levels, and changes in potassium distribution across the cell membrane. Hypomagnesemia occurs frequently with DTs and may predispose to withdrawal seizures [8]. Hypophosphatemia may occur due to malnutrition, may be symptomatic, and if severe, may contribute to cardiac failure and rhabdomyolysis. |
| It is established that electrolyte abnormalities result in different clinical conditions. Decreased blood plasma osmotic pressure as a result of hyponatremia causes different disorders of central nervous system, seizures [22]; |
| hypokalemia may result in different cardiac rhythm disorders, cardiomyopathia and paralyticileus ([22], [23], [24]); hypomagnesemia causes cardiac arrhythmias, mental and emotional changes [24]. So, electrolyte evaluation and appropriate correction must be done during management of chronic alcoholic patients in intensive care units, especially those with alcohol withdrawal. |
| Potassium: Normally the kidneys are a major route of potassium ion excretion and serve as an important site of potassium regulation. Alcohol consumption historically is found to reduce the amount of potassium excreted by the kidneys [25], although the body’s hydration state may help determine whether potassium excretion will increase or decrease in response to alcohol. Levels of potassium, like those of sodium, also can affect the way the kidneys handle fluid elimination or retention. In addition, potassium depletion has been proposed to exacerbate hyponatremia through any of several mechanisms [26]. |
| Sodium :Alcohol does appear to directly influence the kidney’s handling of sodium and other electrolytes, potentially resulting in hypernatremia. In a study by Rubini and colleagues, 1955 [25], subjects who consistently drank about 4 ounces(oz) of 100-proof bourbon whiskey experienced decreased sodium, potassium, and chloride excretion (i.e., increased retention of solutes). Although some exceptions exist, several historical studies have reported similar modest reductions in sodium and potassium excretion following alcohol use ([27], [28], [29], [30], [31], [32], [33]). |
| The serum sodium level is determined by the balance of fluid in relation to that of sodium: Not enough fluid in the body results in a sodium concentration that is too high (i.e., hypernatremia), whereas excessive amounts of fluid produce a sodium concentration that is too low (i.e., hyponatremia). Hyponatremia does not constitute merely a biochemical abnormality but most likely has clinical consequences as well (e.g., impaired mental activity, neurological symptoms, and in extreme instances, seizures). |
| Phosphate: Another potential cause of hypophosphatemia in alcoholic patients is hyperventilation, which can occur during alcohol withdrawal. Prolonged rapid, shallow breathing results inexcessive loss of carbon dioxide and decreased blood acidity (i.e., alkalosis), which in turn activates an enzyme that enhances glucose breakdown. In glucose breakdown, phosphate becomes incorporated into various metabolic compounds, ultimately lowering blood levels of phosphate. As the rate of glucose breakdown increases, profound hypophosphatemia potentially can result. Alcoholic patients also may develop low blood levels of phosphate by excreting too much of this ion into their urine. Alcohol can induce abnormally high phosphate levels (i.e., hyperphosphatemia) as well as abnormally low levels. In fact, hyperphosphatemia often precedes hypophosphatemia. Alcohol consumption apparently leads to excessive phosphate levels by altering muscle cell integrity and causing the muscle cells to release phosphate. This transfer of phosphate out of muscle cells and into the bloodstream results in an increased amount of phosphate passing through the kidneys’ filtering system. |
| There are a number of published reports showing that serum potassium concentration falls during alcohol withdrawal, especially if complicated by delirium tremens ([34], [35]). Some studies even show evidence that potassium could be useful as an indicator to predict delirium tremens ([7], [36]). |
| Magnesium: Chronic alcoholism is the leading cause of low blood levels of magnesium (i.e., hypomagnesemia) [26]. Often it occurs simultaneously with phosphate deficiencies, also frequently encountered among alcoholic patients. Several alcohol-related mechanisms can result in hypomagnesemia. Studies historically have shown that alcohol consumption markedly increases magnesium excretion in the urine and may affect magnesium levels in other ways as well. For example, when rats are given alcohol, they also require significant magnesium in their diets, suggesting that alcohol disrupts absorption of this nutrient from the gut. Investigators have speculated that alcohol or an intermediate metabolite directly affects magnesium exchange in the kidney tubules [26]. |
| Calcium: Early studies showed that alcohol consumption markedly increases calcium loss in urine. In severely ill alcoholic patients, low blood levels of calcium occur about as often as low blood levels of phosphate and can cause convulsions or potentially life-threatening muscle spasms when respiratory muscles are involved. Alcoholic patients with liver disease often have abnormally low levels of a calcium-binding protein, albumin, and also may have impaired vitamin D metabolism; either of these two factors could result in reduced blood levels of calcium (i.e.,hypocalcemia). Muscle breakdown and magnesium deficiency are other potential causes of hypocalcemia in alcoholic patients. A direct effect of alcohol in reducing calcium levels is suggested by at least one experimental study: Dogs became hypocalcemic after administration of alcohol above a critical threshold amount of approximately 1 g/kg [37]. |
| Conclusion: From the data obtained, it is concluded that post-treatment and pre-treatment with ginger extract produced a significant anti-nephrotoxiic effect. Furthermore, ginger is capable of improving hyperlipidemia and the impaired kidney functions in ethanol induced- withdrawal rats maintaining proper kidney functioning as well as maintaining electrolyte balance preventing retention of solutes. |
| Despite the significance of alcohol’s effects on the kidney, however, relatively few recent studies have been conducted to characterize and elucidate their pathophysiology. More studies are required in animals and humans on the kinetics of ginger and its constituents relating the effects of ginger consumption over a long period of time during nephrotoxicity in cases of alcohol abstinence. It is hoped that future investigations will focus on this important subject area. |
| Acknowledgement: The corresponding author sincerely thank Dr.K.Sathyavelu Reddy, Professor, Sri Venkateswara University, Tirupati and Dr.W.Rajendra, Professor, Sri Venkateswara University, Tirupati for the kind support and timely help, for allotting valuable time for healthy discussions and consultation regarding the problem under investigation and also for providing laboratory aid. |
| Conflict of Interest: None Declared. |
References |
|