ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
| Anupriya Sachar1, Sheetu Raina2 Ph.D., Deptt. Of Zoology, University of Jammu, J&K, India1,2 |
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
In the present studies, an attempt has been to analyse the effect of lindane on immune organs of fish, Aspidoparia morar. The fish was exposed to different sublethal concentrations of lindane (10%, 20% and 30% of LC50 value of lindane) for the period of 60 days. LC50 value of lindane for A. morar was found to be 1mg/l. On exposure to lindane, fish has been found to exhibit marked alterations in the cellular architecture (both agranulocytes and granulocytes) of the immune organs viz., anterior kidney, spleen and thymus. These alterations ultimately become the cause of hampering the entire metabolic machinery thereby affecting the physiological status of the fish.
Keywords |
| Lindane, Immune organs, Cellular architecture, Aspidoparia morar |
INTRODUCTION |
| Human destructive influence on the aquatic environment in the form of sublethal pollution usually results in chronic stress conditions that have negative influence on aquatic life [1]. Freshwaters are highly vulnerable to pollution since they act as immediate sinks for the human activities. The two basic developmental indices of a rapidly growing human population are industrialization and improved agricultural productions. These activities are however of major concern in creating a situation of xenobiotic overload in aquatic ecosystems. The most prevalent xenobiotics arising out of industrial and agricultural activities are pesticides and heavy metals where the stress response mechanisms have been widely addressed in vertebrates in general and fish in particular. Fishes mostly have a tendency to bioaccumulate such type of xenobiotics and humans can be at great risk being at the top of the food chain [2]. The objective of the present study was to evaluate the effect of long-term sublethal concentrations of lindane on immune organs viz., anterior kidney, spleen and thymus of A. morar when exposed to different concentrations of lindane (10%, 20% and 30% of LC50 value of lindane) The paper is organised as follows. Section II describes the methodology adopted for the research work. Section III represents the effect of stressor (lindane) on anterior kidney, spleen (Section III a) and thymus (Section III b) in the form of results and discussion. Section IV reflects the conclusion part of the research work. |
II. MATERIALS AND METHODS |
| For studies related to cellular architecture of immune organs viz., anterior kidney and spleen imprinting method was employed. Freshly cut pieces of immune organs (viz., anterior kidney and spleen) were gently blotted on a clean slide and an imprint was made of the resident cells. Slides so prepared were air dried and stained using leishman-geimsa stain [3] and cellular components were identified using compound microscope. Since thymus is not a localised organ, it was not possible to evaluate the alterations in its cellular composition by imprinting method. Therefore the immune cells of thymus were studied by histological method. For histology, thymus tissue was embedded in histowax of 54-56°C. 5-7 μm thick sections were cut on microtome and stained using haematoxylin eosin stain. Slides of immune organs were scanned and photographed with Sony-DC378P-Semi-Digital Camera attached with Olympus CH20i Research microscope. |
III. RESULTS AND DISCUSSION |
| a) Effect of lindane on anterior kidney and spleen |
| Presently, fish A. morar when exposed to different sublethal concentrations of lindane, exhibited various alterations in the immune organs at the cellular level (Figs. 1-12). Control group of fishes (Figs. 1, 2 and 7, 8) showed the presence of various immune cells viz., lymphocytes, monocytes, neutrophils, eosinophils and basophils in addition to macrophages. Figs. 1-12 very clearly reveals that lymphocytes, monocytes and neutrophils observed decline exhibiting thereby lymphopenia, monocytopenia and neutropenia respectively compared to control. Macrophages also were observed to decline in all the treated groups. Decline in all these cells became evident after 10th day of the experimental period of 60 days and become more pronounced as the chronicity of the lindane progressed. This is clearly reflected by alterations in various cellular types of anterior kidney and spleen (Figs. 3 and 9). Eosinophils and basophils on the other hand did not record any change in their values compared to that of control fishes. The lymphopenia observed in immune organs during studies may be attributed to the impairment of proliferation capacity of these cells in the haematopoietic cells of the immune organs. The impairment can seemingly be because of toxicity of insecticide used presently which is known to have suppressive effect on cellular immunity of the fish i.e. suppression of lymphocyte proliferation [4]. Decline in the lymphocyte count of the fish in the immune organs as a consequence of lindane exposure, it appear, inhibits the release of lymphocytes in the general circulation (Fig. 3-6 and 9-12). Similar to present findings, Donaldson [5] and Peters and Schwarzer [6] also held the decline in lymphocyte count of blood to be because of depression of lymphocytes in the immune organs. Numerical decline of monocytes and neutrophils following chronic exposure to lindane appears to affect the phagocytic process of fish . Present observations are in agreement with the result s described by Siwicki et al. [7] who stated that phagocytic activity do get influenced by exposing the fish to various xenobiotics. Immune organs are the sites of macrophage differentiation [3]. The percentage of the macrophages in the treated fishes exposed to different sublethal concentrations of lindane has been observed to record an appreciable decline (Figs. 5, 6, 11 and 12) which rather should have increased under chronic stress of pesticides. Reduction in numerical count of macrophages may therefore be indicative of the fact that stress level of lindane toxicity is so high that instead of increase in their number to enhance phagocytic activity, macrophages itself appear to have got affected to such an extent that it no longer remains possible for fish to withstand the severity of the stress. Such condition, if further prevails can even lead to mortality of fishes. Similar macrophage related suppression of phagocytic activity in immune organs under the influence of xenobiotics have earlier also been reported by Stephan and Susanne [8]. Eosinophils and basophils are recognised as responsible candidate which can contribute to phagocytosis. In the treated fishes during the experimental period of 60 days no notable difference could ever be observed concerning both eosinophils and basophils between the exposed and unexposed fishes (Figs. 3-6 and 9-12). This according to the present author can be explained on the pretext that since these cells are present in very low percentages (3-5%), their contribution to phagocytic process either is negligible or at the most may be to substantiate the function of macrophages [9]. Thrombocytes are the blood constituents which plays an important role in blood clotting and phagocytosis [3]. Presently thrombocytes have shown increase in their number (Figs. 5, 6 and 11, 12). Thrombocytosis, present author feels may be an aid in strengthening the phagocytic activity of the fish by which it can overcome the severity of stress. Present results and discussion clearly reveals that toxicity of lindane has an immunedepressive effect on the immune organs viz., anterior kidney and spleen of the fish, A. morar. This immunodep- ressive effect weaken the immune system of the fish to such an extent that it can become prone to various diseases and infections. |
| b) Effect of lindane on thymus tissue: |
| Thymus is a specialized organ of the immune system. The main function of the thymus is the proliferation and maturation of lymphocytes which are critical cells of adaptive immune system. The thymus provides an inductive environment for the development of lymphocytes from haematopoietic progenitor cells [10]. Presently, in fish A.morar, the thymus tissue has been studied by its histological preparation and is characterised by the presence of i) Lymphocytes (Fig. 13) (production of antibodies) and ii) Thymocytes (Fig. 13) (endocrine in function) The thymus tissue of A. morar upon treatment with various sublethal concentrations of lindane (viz., 10%, 20% and 30% of LC50 value of lindane) exhibited various alterations. These alterations included: i) Necrosis observed by 10th day of the experiment and become more pronounced on day 30th (fig. 14, 15) ii) vacuolation of the thymus tissue observed at 50th day of the experiment (Fig. 16) followed by iii) total degeneration (Fig. 17) towards the end of experimental period of 60 days. Similar to present findings, Toursaint et al. [11] in Japanese medaka (Oryzias latipes) and Grinwis et al. [12] in European flounder (Platicthys flasus) also observed various alterations in the thymus tissue during chronic exposure to complex environmental mixture. They considered these alterations to be the causative for disruption of normal functioning of the thymus tissue under the stressful conditions of xenobiotics. Presently, as a consequence of lindane toxicity necrosis which was observed after 10 days of the experiment implies state of cell death and is the primary cause of tissue degeneration. It causes impairment in the normal functioning of the thymic tissue. Necrosis can lead to the vacuolation of the tissue which actually means the appearance of vesicles/ vacuoles in the tissue and thus results in imbalance in the rate of synthesis of substance and their subsequent release in the general circulation. This disrupts the synthetic machinery of the tissue thereby leading to its total degeneration. Vacuolation as stated by Pal [13] can affect the synthesizing machinery of the tissue ultimately leading to the total degeneration of the tissue. The total degeneration of the thymus tissue leads to decline in immunocompetence of the fish. |
IV. CONCLUSION |
| Lindane, presently have been found to significantly affect the immune organs of the fish thereby affecting the entire physiology. This organic pollutant in the natural water bodies (after their runoff from the agricultural fields) definitely appear to affect various metabolic processes and hence general health status of the fish. Therefore, it is high time that aquatic ecosystem be safeguarded against inflow of such inadvertently released toxicants, which through their inflow in the aquatic ecosystem result in a buildup of their concentration to such high level that they start deleteriously affecting the abiotic and biotic system operating in the aquatic environment which indirectly have an adverse effect on human health through food chain. |
ACKNOWLEDGEMENTS |
| I am very thankful to Department of Zoology, University of Jammu, Jammu for providing the necessary facilities regarding the research work. |
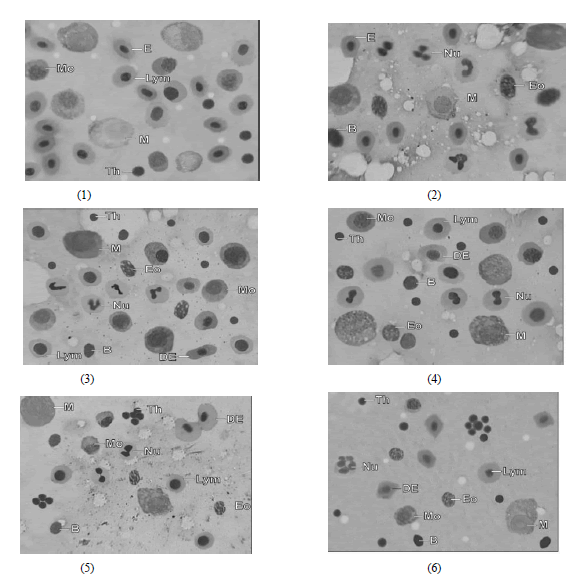 |
| Fig. 1 and 2 Microphotograph of imprint of kidney tissue of A. morar from control showing normal Erythrocytes (E), Lymphocytes (Lym), Monocytes (Mo), Macrophages (M), Neutrophils (Nu), Eosinophils (Eo), Basophils (B) and Thrombocytes (Th) |
| Fig. 3-6 Microphotograph of Imprint of kidney tissue of A. morar treated with lindane showing Distorted Erythrocytes (DE), decline in Lymphocytes (Lym), Monocytes (Mo), Neutrophils (Nu), Macrophages (M) with increase in thrombocyte count of the fish after 10, 30, 50 & 60 days of the experiment respectively. Eosinophils (Eo) and Basophils (B) didnot show any notable change |
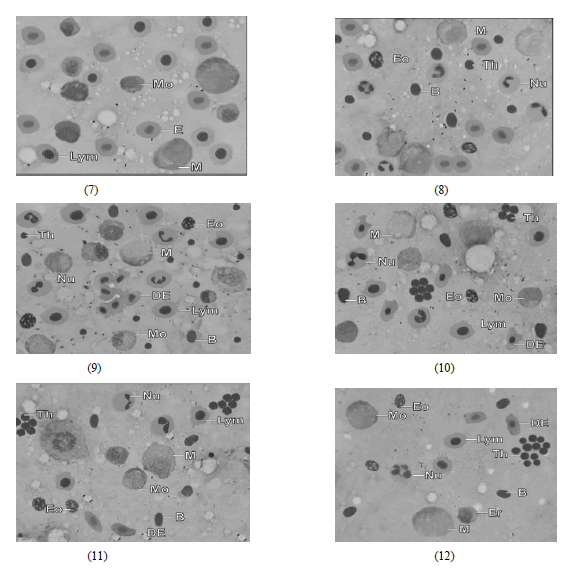 |
| Fig. 7 and 8 Microphotograph of imprint of Splenic tissue of A. morar from control showing normal Erythrocytes (E), Lymphocytes (Lym), Monocytes (Mo), Macrophages (M), Neutrophils (Nu), Eosinophils (Eo), Basophils (B) and Thrombocytes (Th) |
| Fig. 9-12 Microphotograph of Imprint of Splenic tissue of A. morar treated with lindane showing Distorted Erythrocytes (DE), decline in Lymphocytes (Lym), Monocytes (Mo), Neutrophils (Nu), Macrophages (M) with increase in thrombocyte co unt of the fish after 10, 30, 50 & 60 days of the experiment respectively. Eosinophils (Eo) and Basophils (B) didnot show any notable change |
 |
| Fig.13 Microphotograph of Thymus tissue of A. morar from control showing Lymphocytes (Lym) and Thymocytes (Th) |
| Fig. 14-17 Microphotograph of Thymus tissue from lindane treated fish A. morar showing Necrosis (N), severe Necrosis (N), Vacuolation (V) and Total degeneration (TD) of the tissue after 10, 30, 50 and 60 days of the experiment respectively. |
References |
|