ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Sarita Sharma1, Rachana Bhatt2
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
In the present study, different soil samples were analyzed to find a potent amylase producer. We found a natural soil isolate, named as Pseudomonas sp. B5 – a Gram negative short rod, which gave maximum amylase production (12.79 μmole/ml/min) in production medium containing 3% soluble starch when incubated for 24 h at 150 rpm. Further characterization of partially purified amylase proved it to be an enzyme having molecular weight of 55,370 KDa with 1.8 μmole Km and 0.019 U/min Vmax. Enzyme showed pH optima of 5 with activity in wide range of temperature ranging from 30 oC - 100 oC. This amylase was stable up to 35 minutes at 100 oC. Effect of several inhibitors and metal ions on amylase activity was also checked. EDTA proved to be detrimental for the amylase where as presence of metal ions namely Ca+2 and Fe+3 showed increase in the activity of the amylase.
I. INTRODUCTION |
| Amylases are among the most important enzymes and are of great significance for biotechnology, constituting a class of industrial enzymes having approximately 25% of the world enzyme market. Alpha-amylases (1, 4-D-glucan glucanohydrolase, E.C.3.2.1.1) breaks down long-chain carbohydrates by acting at random location along the starch chain, ultimately yielding maltotriose and maltose from amylose, or maltose, glucose and “limit dextrin” from amylopectin (Das et al., 2011). With the advances in biotechnology, the amylase application has expanded in many fields such as clinical, medicinal and analytical chemistry, as well as their widespread application in starch saccharification and in the textile, food, paper, detergent, pharmaceutical brewing and distilling industries. Thermostability is a desired characteristic of most of the industrial enzymes. Thermostable enzymes isolated from thermophilic organisms have found a number of commercial applications because of their stability. As enzymatic liquefaction and saccharification of starch are performed at high temperatures (100-110 ºC), thermostable amylolytic enzymes have been currently investigated to improve industrial processes of starch degradation and are of great interest for the production of valuable products like glucose, crystalline dextrose, dextrose syrup, maltose and maltodextrins. In this paper, we have isolated and characterized one such thermostable amylase from a natural soil isolate. |
II. MATERIAL AND METHODS |
MATERIALS |
| Soluble starch, Glucose, Dinitrosalicylic acid (DNSA), Folin’s Reagent, Acetate Buffer, Gram’s reagents, Bovine Serum Albumin (BSA). |
| Medium Used: Nutrient broth, Nutrient Agar, Starch agar plate and 100 ml Production medium consisting of Soluble Starch – 1.2 gm, Beef Extract – 0.3 gm, Agar – 3 gm, pH – 7.5. |
2.1 Isolation of amylase producing microorganism |
| For the isolation of amylase producing organism having the ability to degrade starch, soil samples from different sites were taken. Loop-full of the soil sample was streaked on starch agar plate and incubated at 37 ºC for 24 h. After 24 h of incubation at 37 ºC, single colonies of different sizes were selected. Colonies which formed clear halos after addition of Gram’s iodine were identified as starch degrading strains. The culture having maximum zone of degradation was selected for further studies. |
2.2 Identification of the starch degrading organism |
| Culture was subjected to Gram Staining and various biochemical tests for identification. |
2.3 Inoculum development of amylase producing organism |
| For inoculum development, 50 ml N-broth in 100 ml flask was prepared and autoclaved 15 psi for 15 minute. The culture showing highest starch degradation was inoculated in this broth and the flask was then incubated on shaker at 150 rpm for 24 h. After 24 h of incubation 5 ml of this broth was centrifuge at 10,000 rpm for 10 minute. Growth was measured in terms of O.D. of the pellet at 660 nm. Production media was then inoculated by adjusting 1 O.D. /100ml of medium. |
2.4 Production of amylase |
| The inoculum developed as above was then inoculated in the production media so as to get final 1 O.D.660/100 ml of the medium. The production flasks were kept on environment shaker at 150 rpm. Samples of 5 ml were withdrawn from production medium after 24, 48 and 72 h respectively. These samples were then centrifuged at 10,000 rpm for 10 minute. Supernatant was further used for amylase assay and protein estimation. Amylase thus produced was an extracellular enzyme. |
2.4.1 Amylase assay |
| For enzyme assay the reaction mixture containing 1 ml crude enzyme (supernatant) and 1 ml solution of soluble starch (1%) was incubated at 37 ºC for 15 minute. The reaction was stopped by addition of 1 ml dinitro-salicylic acid (DNSA). The tubes were boiled for 10 minute. After cooling the tubes and add 7 ml distilled water. Absorbance was taken at 540 nm and amount of reducing sugar (glucose) was calculated from standard graph of glucose (1 mg/ml). This protocol is referred to as standard protocol from here. One unit of amylase activity is defined as the amount of enzyme that releases one μmole of glucose from soluble starch under above assay condition. |
2.4.2 Protein estimation |
| Protein concentration was determined by Folin-Lowry’s (Lowery et al., 2005) method, with bovine serum albumin as standard (200μg/ml). |
2.5 Optimization of cultural parameter |
| Optimization of various parameters and development of media are the most important criteria for the overproduction of the enzyme. Several parameters studied for optimization of enzyme production are as follows: |
2.5.1 To study the effect of different incubation time on amylase production |
| Inoculum was prepared as described above. Production media flasks containing 100 ml media was inoculated with inoculum at 1 O.D.660/100ml. The production flasks were then incubated on shaker at 150 rpm. Maximum amylase producing ability of the organism was checked by harvesting the sample at 24, 48 and 72 h respectively. |
2.5.2 To study the effect of different inoculum size on amylase production |
| Prepare the inoculum as described above. Inoculate the production medium at different inoculum size; 0.5 O.D./100 ml, 1 O.D./100 ml, 1.5 O.D./100 ml, 2 O.D./100 ml. Samples were harvested at 24, 48 and 72 h. Amylase production was analyzed by standard protocol. |
2.5.3 To study the effect of different concentration of starch on amylase production |
| Prepare the inoculum as described above. Inoculate the production medium having a different concentration of starch; 0.5%, 1%, 1.5%, 2%, 2.5% and 3%. Analyze the amylase production in different flasks. |
2.5.4 To study the effect of different pH on amylase production |
| Effect the different pH on amylase production was studied by inoculating the production medium having the different initial pH of 4, 5, 6, 7, 8 and 9. Samples were harvested at 24, 48 and 72 h. Amylase production was analyzed by standard protocol. |
2.5.5 To study the effect of different temperature on amylase production |
| Effect of different temperatures on amylase production was studied. The production medium was inoculated and incubated at different temperatures i.e. 28 ºC, 37 ºC and room temperature. Samples were harvested at 24, 48 and 72 h. Amylase production was analyzed by standard protocol. |
2.6 Partial purification of amylase enzyme: |
| Entire purification procedure was carried out at cooling condition and in 0.1 mM acetate buffer (pH 5) throughout unless otherwise specified. |
2.6.1 Preparation of crude enzyme |
| Amylase was produced using the optimum conditions for the production. The production medium was harvested and centrifuged at 10,000 rpm for 10 min at 4 ºC. The supernatant thus obtained was considered as crude enzyme. |
2.6.2 Ammonium sulfate precipitation |
| For the precipitation of enzyme from the supernatant ammonium sulfate precipitation method was used. Ammonium sulfate was added to the enzyme supernatant to get 0-20%, 20-60%, 60-80% and 80-100% saturation. After each saturation step, medium was centrifuged at 10,000 rpm for 10 minute at 4 ºC and precipitates thus obtained were dissolved in a minimum volume of a 0.1 mM acetate buffer (pH 5) so as to dissolve the pellet. The supernatant was then used for subsequent saturation step. |
2.6.3 Dialysis |
| The dialysis bag was used for removal of salts with the help of 0.1 mM acetate buffer (pH 5). Conventional use of dialysis bags involved the removal of unwanted low-molecular-weight solute from the sample and replaced with buffer present in the “dialysis”. Each fraction of ammonium sulfate saturation was taken in separate dialysis bags. These fractions were dialyzed overnight against the acetate buffer under cooling condition. In the entire process of dialysis, the buffer was changed thrice. |
2.6.4 SDS-PAGE |
| Sodium dodecyl sulfate-polyacrylamide gel electrophoresis was used for separation of protein on the basis of size and molecular weight. Electrophoresis was carried out at room temperature using Laemmli buffer system (Laemmli, 1970) and by using protein molecular weight marker Broad range (Medox-Biotech Indian Pvt. Ltd., India). |
2.7 Characterization of enzyme |
2.7.1 Enzyme kinetics |
| Aliquotes of stock solution (1%) were taken so as to get different substrate concentrations (100 μg - 1000 μg) in 2 ml assay system containing 1 ml of purified Enzyme and water so as to make the final volume (2 ml) of the assay system. The reaction was carried out at 37 ºC. Km and Vmax of the Enzyme was obtained from the Lineweaver-Burk double reciprocal analysis. |
2.7.2 Effect of pH and temperature on amylase activity |
| The optimum conditions for enzyme activity were determined using partially purified enzyme by DNSA assay as described before. For determination of effect of pH on enzyme activity, take 1ml substrate (1%) and 1 ml enzyme , 0.5 ml of 0.1mM buffer: acetate buffer (pH 3.0 - 5.0), phosphate buffer (pH 6.0 - 8.0) and incubate at 37 ºC. The effect of temperature on enzyme activity was determined in the range of 30 - 120 ºC. Water bath was used to maintain the temperature of the assay system. |
2.7.3 Effect of different incubation time on amylase activity |
| The optimum conditions for enzyme activity were determined using purified Enzyme by DNSA assay as described before. For determination of effect of different incubation time on enzyme activity, take 1 ml substrate (1%) and 1 ml enzyme and incubate it for different time intervals ranging from 5-30 minutes at 100 ºC (optimum temperature). |
2.7.4 Effect of inhibitors, metal ions on amylase activity |
| Effect of inhibitors (10mM) namely; sodium dodecyl sulfate (SDS), HgCl2, sodium arsenate, urea, ethylenediaminetetraacetate (EDTA), on amylase activity was checked. Effect of different metal ions (10mM) namely; Ca+2, Mg+2, Mn+2, Zn+2, Fe+2, NH4 +2 on amylase activity was also checked. |
III. RESULTS AND DISCUSSION |
3.1 Isolation of starch utilizing organism |
| For initial screening of starch utilizing organisms, two different soil samples were collected and inoculated in starch broth having 1% starch. The flasks were incubated on environment shaker at 150 rpm for 24 h. Starch utilizing microbial colonies were obtained by streaking the enriched broth on starch agar plates which were incubated at 37 ºC for 24 h. Different starch utilizing organisms showed starch degradation zones. Amylase production was confirmed on starch agar plates by flooding the plates with iodine solution. Initially the plates acquired deep blue color, after few second, the deep blue color around some bacterial colonies started showing clear zone which confirmed extracellular secretion of amylase from the bacteria strain followed by starch degradation. One isolated bacteria showing highest enzymatic activity was further selected for studies. |
3.2 Morphological characteristic and Biochemical Identification Analysis |
| The culutre was a Gram negative short rod and from the preliminary identification tests it was confirmed to belong to Pseudomonads family and is referred to as Pseudomonas sp. B5. |
3.3 Optimization of cultural parameter |
| Various physical and chemical factors have been known to affect the production of amylase such as incubation time, inoculum size, pH, temperature, different starch concentration etc. |
a) To study the effect of different incubation time on amylase production |
| To optimize the incubation time for maximum amylase production sample was harvested at 24 h interval for 72 h from the production medium and centrifuged at 10,000 rpm for 10 minute. Supernatant was used for the amylase assay as described in methods and protein estimation (Lowery et al., 2005). The growth of culture was also checked in terms of O.D. The results obtained are shown in the following graph (Fig.). From graph it was concluded that maximum amylase production (0.41μmole) was observed at the end of 24 h and it decreased with increase in time. |
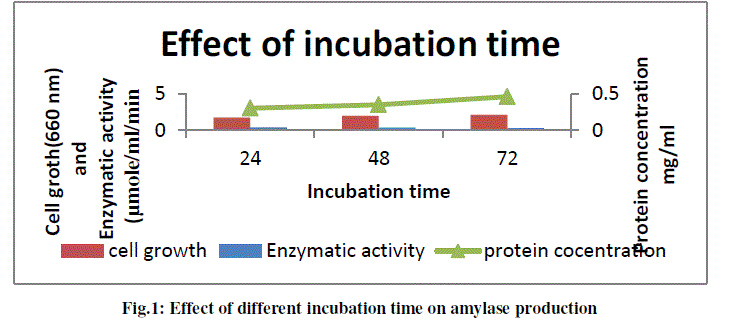 |
| b) To study the effect of different inoculum size on amylase production |
| Effect of inoculum size for maximum amylase production was checked. Different inoculum size such as 0.5 O.D.660/100 ml, 1 O.D. 660/100 ml, 1.5 O.D. 660/100 ml, 2 O.D. 660/100 ml was inoculated in production medium and sample was harvested at the interval of 24, 48, 72 h. The results obtained are shown in the following graph (Fig.2). Since maximum enzyme production was obtained at 24 h, graphical representation of enzyme and protein production at the end of 24 h only is depicted. |
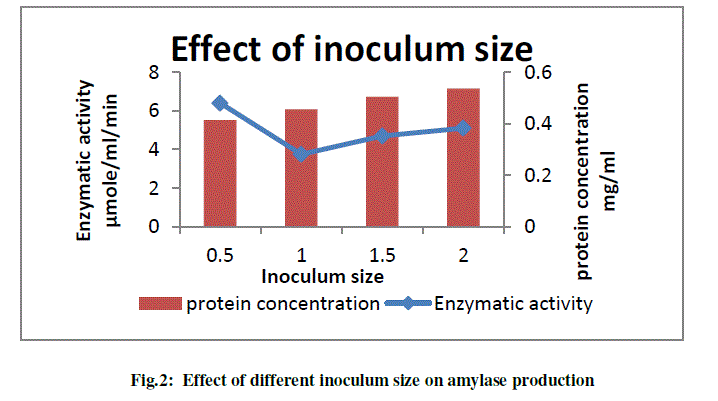 |
| From graph it was concluded that inoculum size of 0.5 O.D./100 ml is optimum for maximum enzyme production (6.40 μmole/ml/min). With increase in inoculum, increase in the extracellular protein content was observed but similar pattern was not observed for the amylase production. |
c) To study the effect of different concentration of starch on amylase production |
| Bajpai et al.,(1989) reported that carbon source greatly influence amylase production and most commonly used substrate is starch. In this paper, the effect of different concentration of soluble starch on amylase production was studied, for this study production medium having different concentration of starch were prepared. Our strain shows a stable increase in the enzyme production with increase in starch concentration. Highest amount of amylase (6.77 U) was produced with 3% starch in production medium (Fig.3). |
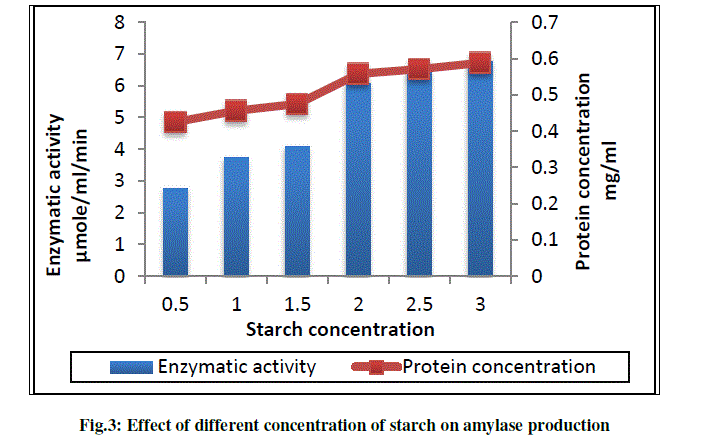 |
d) To study the effect of different pH on amylase production |
| Production media was set at different initial pH in order to check the effect of pH on amylase production by Pseudomonas sp. B5. Different initial pH ranging from 4 to 9 was tested. Our strain gives highest enzymatic activity at pH 5 and yield good enzyme at initial pH range 5 to 7. The results are as shown in the following graph (Fig.4). |
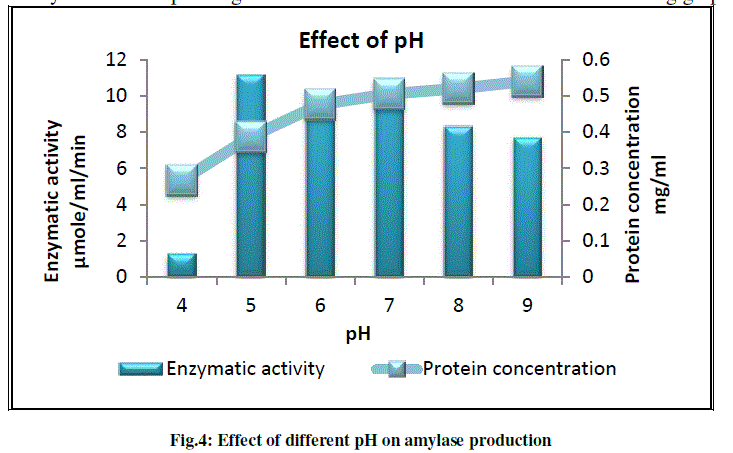 |
| Bajpai et al.(1989)reported that growth of Bacillus licheniformis TCRDC-B13 occurred at pH 3 to 11, although bacterial growth start to decrease as the pH increases. They also found that optimum enzyme production was obtain at pH 6 to 9. Bacterial cultures such as B.subtilis, B.licheniformis and B.amyloliquefacience required an initial pH of 7.0 (Syal et al., 1986). Rhodothermus marnies was reported to yield good enzyme levels at initial pH ranges 7.5 to 8 (Carlsen et al., 1996). |
(e) To study the effect of different temperature on amylase production |
| The effect of temperature on bacterial growth and amylase production from Pseudomonas sp. B5 was studied. The production of enzyme was determined at different temperature; 28 0C, 37 0C and room temperature (32 0C) and optimum enzyme production was observed at room temperature. The results are as shown in the following graph (Fig.5). |
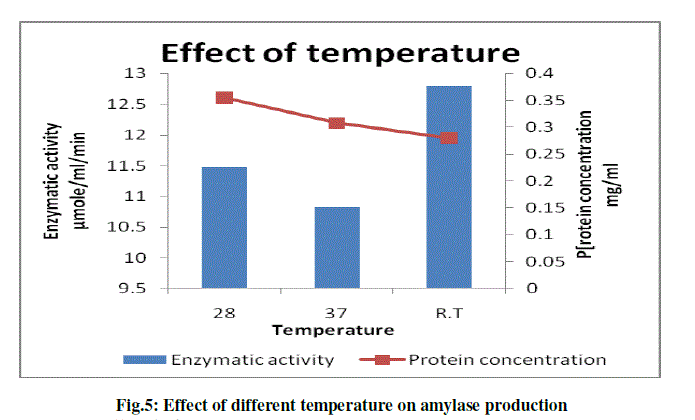 |
| Saito et al., 1973 studied a Bacillus licheniformies strain which produced amylase at temperature around 50 0C and never produced the enzyme at temperature below 45 0C but Pseudomonas sp. B5 showed highest amylase production and cell growth at room temperature (11.45 μmole/ml/min), enzyme production was also observed at 28 0C (9.09 μmole/ml/min) and 37 0C (9.78μmole/ml/min). |
3.4. Partial purification procedure of amylase enzyme |
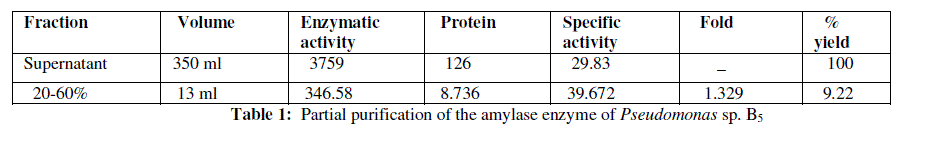
| A two step protein purification protocol was followed. These two steps included; total protein precipitation from the broth using ammonium sulphate precipitation and followed by Dialysis for removal of salts with the help of 0.1 mM Acetate buffer. Amylase produced by Pseudomonas sp. B5 was only partially purified enzyme with an overall yield of 9.22% at the end of the 60% saturation of the supernatant by ammonium sulphate. A purity fold of 1.32 (Table 1) was achieved at the end of 60% ammonium sulphate precipitation step. |
 |
3.5. Characterization of amylase: |
3.5.1: Activity staining and molecular weight determination of the partially purified amylase |
| Native PAGE was done in order to find the exact location of amylase on the gel. |
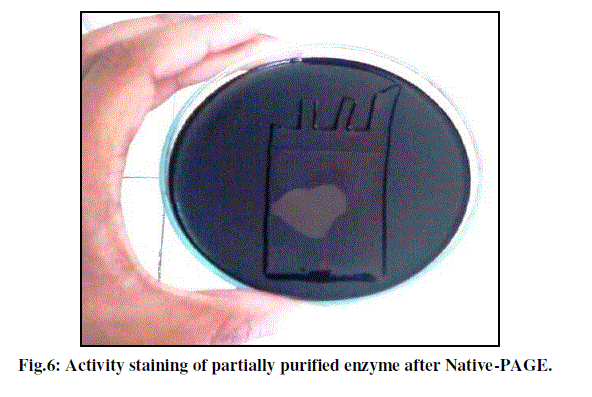 |
| Figure 6 shows the activity staining of the gel after performing the native electrophoresis. Clear zone of hydrolysis of starch is visualized after an incubation of native electrophoresis gel for 10 minutes on starch agar plate confirming the presence of amylase in the loaded sample and its location on the polyacrylamide gel. |
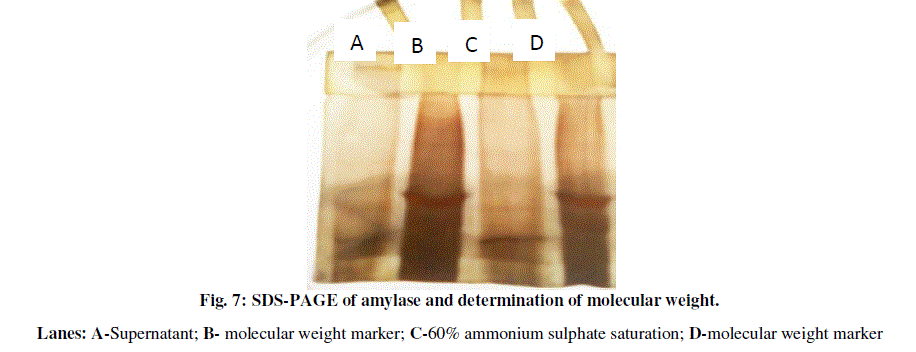
| For molecular weight determination SDS-PAGE was done. Partially purified enzyme sample showed presence of approximately four bands on denaturing polyacrylamide gel (Fig. 7). The molecular weight of the purified PHB depolymerase was found to be around 55,350 KDa when compared on the basis of the Rf values (Table 2). |
 |
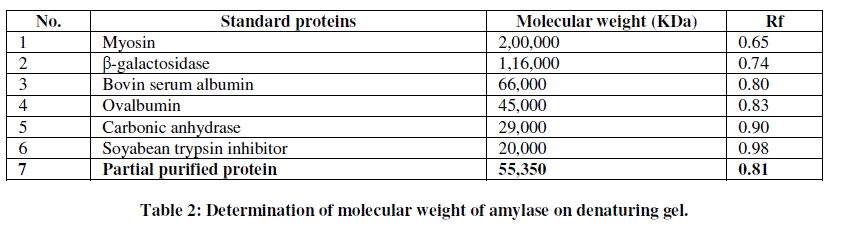 |
| The molecular weight of the amylase is around 55,370 KDa which was further found to be stable at 100 ºC. |
3.5.2 Enzyme kinetics |
| The properties of amylase such as thermostability and pH profile are necessary to be elucidated in order to have its industrial application. Hence, the diversity of the application creates the need to search for novel amylase with novel and improved properties. The rate of hydrolysis of starch by amylase depends on many process conditions such as temperature, pH, and nature of substrate, substrate concentration, and enzyme concentration. Hence the partially purified enzyme was further characterized. |
a) Effect of different starch concentration on amylase activity of the partial purified enzyme |
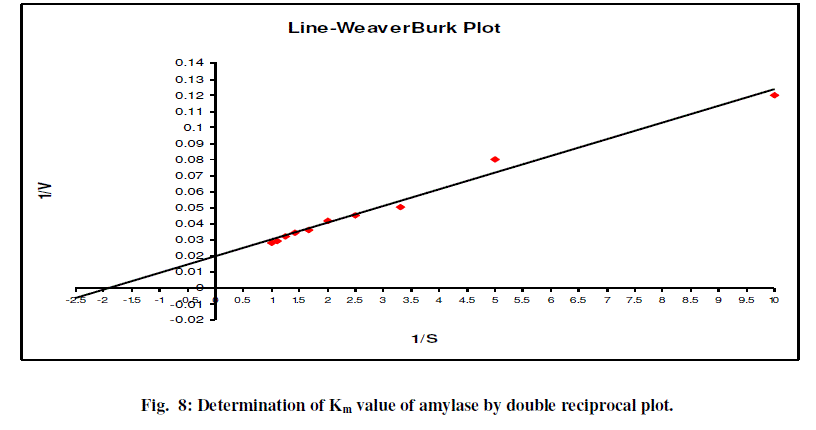
| Lineweaver – Burk plot (Fig.8) showed the Km value of amylase to be 1.8 μmole and Vmax value is 0.019 U/min. |
 |
| b) Effect of pH and temperature on partially purified amylase activity |
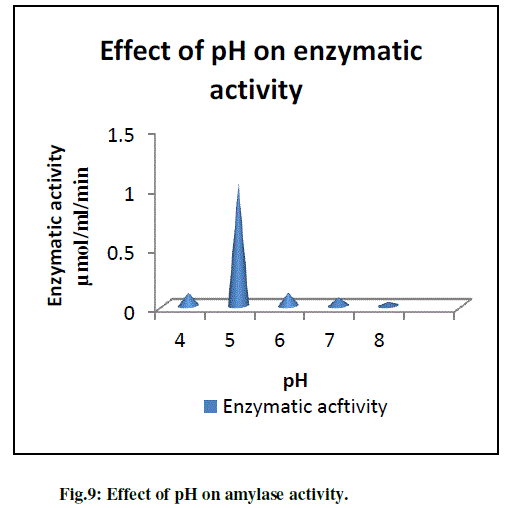
| The purified amylase was active in the acidic pH range from pH 4.0 to pH 5.0 (Fig.9). The optimum activity was obtained in buffer (0.1M Acetate buffer) pH 5.0. From graph it was concluded that initial enzymatic activity decrease at pH 4 with increase in the enzymatic activity at pH 5 and subsequently decrease. So amylase gives the maximum enzymatic activity enzymatic activity at pH 5. The optimum activity of the enzyme at low pH values (pH 4.5-5.0) with a good stability at pH-5.0 is very important from the view point of industrial application (Sajedi et al., 2005). |
 |
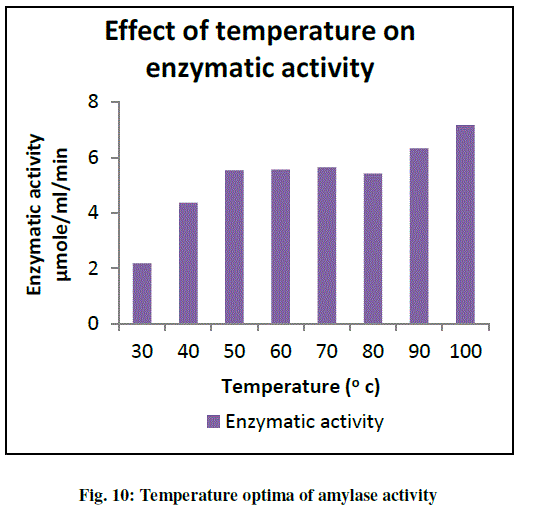
| Effect of different temperature on enzyme activity was studied by incubating the enzyme and substrate at temperature range such as 30 º to 100 oC. The results are as shown in graph (Fig. 10). Temperatures ranging from 30 oC to 100 oC supported the enzyme activity with the maximum activity at 100 oC (Fig. 10). It is desirable that amylase should be active at high temperature for gelatinization (100-110 ºC) and liquefaction (80-90 ºC) to economize the process. Thus this partially purified enzyme has been proved to be commercially important enzyme having applications in the areas where higher temperatures are required. From the graph it was found that Pseudomonas sp. B5 gave maximum enzymatic activity at 100 ºC. The purified amylase showed activity in wide range of temperature thereby making this culture a potent organism to be used for application in industrial applications where high temperatures are needed. Along with activity at high temperature the thermal stability is equally important. This property could be favorable in industrial operation for traditional brewing and food processing (Stamford et al., 2001). Hence further characterization of the enzyme was done in terms of its thermal stability. This was done by incubating the enzyme at higher temperatures for a long period of time and then checking its activity. |
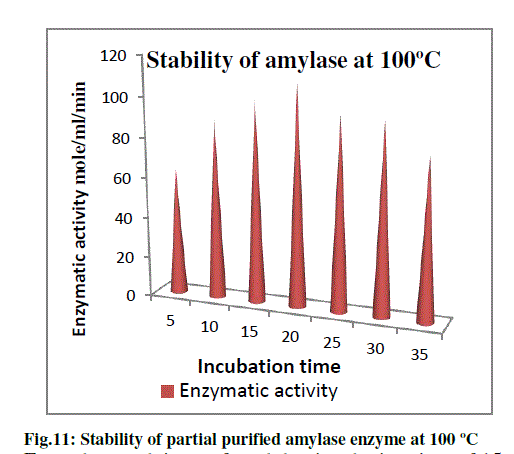
c) Thermal Stability of amylase at 100 oC: |
| In order to check the thermal stability of amylase produced by Pseudomonas sp. B5, partially purified enzyme was first kept at 100 oC in water bath and aliquot of 1 ml was taken at every 5 minutes interval and then enzyme assay was done at 100 oC. The results obtained are as shown in the graph (Fig.11). From the graph it can be seen that the enzyme gave maximum activity (7.2 U) at around 20 minutes. However after that the activity started diminishing. But partially purified enzyme showed considerable activity and hence thermal stability even after being kept at 100 oC for 35 minutes. One point that is of consideration is that the incubation time in the enzyme assay is also 15 minutes which is also done at 100 oC. Thus partially purified enzyme has shown good thermal stability and thus could be said as industrially important enzyme. |
d) Effect of different incubation time on enzyme activity: |
| Effect of Different incubation time on enzyme activity was studied by incubating the enzyme and substrate for 5, 10, 15, 20 and 25 minute. The results are shown in the graph Fig 12. It was found that incubation time of 15 minute is optimum for enzyme activity. |
 |
e) Effect of different inhibitors on enzyme activity |
| The effect of metal chelators and chemical compounds helps to study the characteristic of the enzyme. The effect of inhibition of metal chelators indicates the requirements of certain metal ions for enzyme activity. These was studied by adding the inhibitor such as HgCl2(10mM), sodium arsenate(10mM), urea(10mM), SDS(10mM), EDTA(10mM) with enzyme-substrate solution and find out the relative activity. Results are shown on graph Fig.13. We have received some outstanding results by this analysis. The amylase produced by Pseudomonas sp. B5 has shown relatively high activity in presence of HgCl2, Sodium arsenate, Urea as well as SDS. Those chemical proved to be inhibitors for many of the enzymes are ineffective for this amylase. However, these results are yet to be further explored. EDTA decreased the activity of the enzyme that proves the need of specific metal ions for the activity of the amylase which when chelated by EDTA resulted into decrease in the activity. To find the presence of as to which metal ion increases the activity of the amylase produced by Pseudomonas sp. B5 we then further thought of studying the effect of different metal ions on the amylase activity. |
f) Effect of different metal ions on enzyme activity |
| Most of amylase are known to be metal ion-dependent enzyme, namely divalent ion like Ca2+, Mg2+, Mn2+, Zn2+, Fe2+, NH4 2+ etc (Panday et al., 2000). Effect of that metal was studied by incubating these metal ions with enzyme-substrate solution and find out the % relative activity which are shown on graph. |
 |
| From graph 14 it can be seen that different metal ions have different effect on amylase activity. Ions like Ca +2 and Fe+3 proved to be activity enhancers whereas Mg+2, NH4 +2, Zn+2 as well as Fe+2 proved to be inhibitors for amylase action on starch. Ca2+ was reported to increase α-amylase activity of an alkaliphilic Bacillus sp. ANT6 (Burhan et al., 2003). The stability effect of Ca2+ on thermostability of the enzyme was explained as the salting out of hydrophobic residues by Ca2+ in the protein, thus, causing the adoption of a compact structure (Goyal et al.,2005). |
IV. CONCLUSION |
| Amylase is one of the most industrially explored and important enzyme. In this work amylase produced by a natural soil isolate Pseudomonas sp. B5 was studied in detail. Pseudomonas sp. B5 gave maximum amylase production (12.79 μmole/ml/min) in production medium (pH 5) containing 3% soluble starch when incubated at room temperature, 150 rpm for 24 h. Further characterization of partially purified amylase proved it to be an enzyme having molecular weight around 55,370 kDa with Km of 1.8 μmole and Vmax around 0.019 U/min. Enzyme showed pH optima of 5 with activity in wide range of temperature ranging from 30 to 100 oC. Most outstanding characteristic of amylase produced by Pseudomonas sp. B5 is that it is stable up to 35 minutes at 100 oC and gives considerable activity. Effect of several inhibitors and metal ions on amylase activity proved EDTA to be detrimental. The presence of metal ions namely Ca+2 and Fe+3 showed increase in the activity of the amylase. Pseudomonas sp. B5 can be considered as commercially viable strain and the amylase produced by this strain has wide industrial application due to its property of remaining active in broad range of pH as well as temperature. Thus amylase produced from a natural soil isolate, Pseudomonas sp. B5 is a commercially important amylolytic enzyme. |
References |
|