ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
| N. M. Chavhan* P. G. Department of Chemistry, SSGM College, Kopargaon, Ahmednagar, (M. S.), India |
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
A new series of pyrazoline derivatives (4a-f) has been synthesized by ultrasonication methods and evaluated for its antibacterial and antifungal activity. The chalcones (3a-f) were transformed into respective pyrazoline derivatives by using hydrazine hydrate and few drops of glacial acetic acid in ethanol. All the synthesized compounds were confirmed by FTIR, 1HNMR and mass spectral data.
Keywords |
| Environmental Vulnerability Index, Evaluation model, remote sensing, GIS, Iraq |
INTRODUCTION |
| Recent interest in evaluating environmental vulnerability has been stimulated by increasing awareness that agricultural land is a critically important component of the earth biosphere as it functions not only in the production of food but also in the maintenance of environmental quality as related to fragile-ecosystem management and in formulating and evaluating sustainable agricultural and land use/cover policies [1-3]. In Iraq, environmental vulnerability was not discussed in literature for nearly a decade because the primary emphasis of land management was on controlling soil erosion and minimizing the effects of soil loss on productivity (e.g. [4]). |
| Many methods for environmental vulnerability evaluation have been proposed, such as the comprehensive evaluation method [5], the fuzzy evaluation method [6], the artificial neural-network evaluation method [6], the landscape evaluation method [8-10], and the AHP method [11]. Remote sensing and GIS have evolved into powerful tools for variables acquisition and ecological environment vulnerability spatial distribution study [12-15]. Combing these technologies cannot only supply a platform to support hierarchical integrated analysis on resource and environment, but also integrate the obtained information into a comparative theoretical agricultural land quality analysis [16]. A series of indexes systems have been established. Li [16] developed environmental numerical model using spatial principle component analysis method. The model contains nine factors including elevation, slope, accumulated temperature, drought index, land use, vegetation, soil, water-soil erosion, and population density. Zhang [17] chose dryness, NDVI, geomorphic type and other indexes, and acquired their respective weights by analytic hierarchy process to finish the Eco-environmental vulnerability evaluation of Fuzhou city. Wang [8] analyzed the vulnerability degree of various types of landscape by selecting several indexes such as landscape fragmentation, separation degree, fractal dimension and using of landscape pattern evaluation method. |
| The present study was carried out to assess the environmental vulnerability under different land use/cover and proposed a quantitative formula for assessing environmental vulnerability and suggested that such assessments could help determine how land responded to various management practices. Environmental vulnerability began to be interpreted as a sensitive and dynamic way to document soil condition, response to management, or resistance to stress imposed by natural forces or human uses. The hypothesis is that land properties build up at different land use/cover in very fragile soil is the driving force to resist a high degradation process. |
| The objectives of this research were to assess environmental vulnerability by using the method of integration environmental vulnerability index (EVI) with aid of remote sensing and GIS techniques in Basrah Province, Iraq and explore the influence factors of land use/cover types and soil management practices on spatio-temporal variation of land quality. |
| This article is divided into four sections: following this introduction is a section discussing the employed materials and methods, a description of the study area and the identification of environmental vulnerability indicators. The subsequent section deals with a presentation of the results and an accompanying discussion, while the article ends with some concluding remarks as to the implications raised by the findings for environmental vulnerability control. |
MATERIALS AND METHODS |
MATERIALS: |
Study Area: |
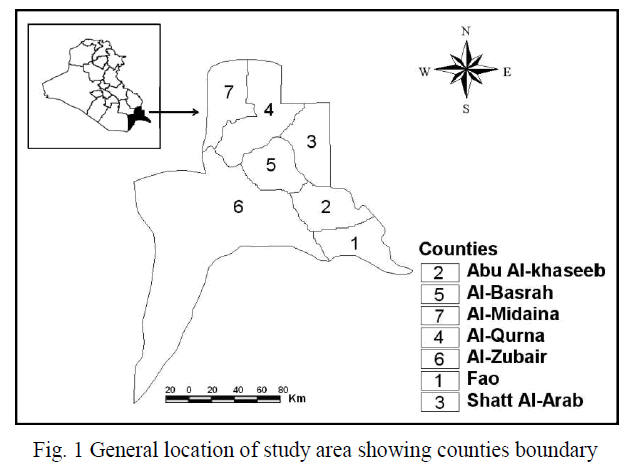
| The study area (Basrah Province), located in the southern parts of Iraq, lies within longitude 46o 60′ to 48o 60′ E and from latitude 29o 13′ to 31o 29′ N with a total area of 19,070 km2 (Fig. 1). To study the land use/cover change, the Districts of Basrah, Abu Al-Khaseeb, Fao, Al Midaina, Al-Qurna, Al-Zubair, and Shatt Al-Arab were selected as a study area. The Districts are situated in the southern parts of Iraq. Average population growth was estimated at 3.6% in the period1990-2003. The soil of Iraq is considered as sedimentary soil, especially in the central and southern parts. The annual humidity is less than 50% and remains less than 30% during the daytime. The average evaporation exceeds 2450 mm/year with average annual rainfall less than 100 mm. The desert plants are adapted to these variations of meteorological factors, and represent 66% of the total cover; these plants begin to grow immediately after rainfall, and complete their life cycle by the end of the rainy period, and soon after the temperature begin to rise. In recent years, along with the increase of population, economic development, improvement of investment environment, Basrah Province has witnessed a rapid urbanization, which reverse has deeply affected ecological environment in the urban and rural regions [18]. |
 |
Remote Sensing Data: |
| Multi-temporal Landsat (WRS2: 165/39, 166/38, 166/39, and 166/40) TM (dated March 1990) and ETM+ (dated March 2003) imageries remotely sensed dataset were assembled and analysed for land use as environmental degradation indicators analysis in the study area. The spatial resolution of one pixel of TM and ETM images were 28.5m by 28.5m. |
Ancillary Data and Software Packages: |
| County-level topographic map, geological map, socio-economic, meteorological data, and all the thematic layers were generated in GIS environment at a scale of 1:250,000. The software packages used for this study were ERDAS for image processing, Arc/Info for analysing and presenting the results, Statistical Graphics, NCSS, and Microsoft Excel were utilised in this research. |
METHODS: |
Pre-processing of images: |
| The pre-processing for the dataset included image registration, radiometric calibration, and radiometric normalisation. Rectification and registration of TM and ETM+ imageries were based on control points collected from vector files of the large and small rivers at the study area using fifty ground control points (GCP). The remotely sensed dataset were geometrically corrected in the datum WGS84 and projection UTM N38 using the first order (linear) of polynomial function and Nearest Neighbour rectification re-sampling, which was chosen in order to preserve the radiometry and spectral information in the imagery [19-20]. Image to image registration was done in order to register the ETM+ image (dated 2003) with geo-coded TM image dated 1990 (master image). The RMS error of the image-to-map was 0.39 to 0.48 pixel, while was 0.18 to 0.24 pixel with image to image registration. The Landsat imageries were radiometric calibrated for sensor differences, converted into spectral radiance and normalized for illumination properties through differences in sun-elevation angle and sun–earth distance by recalculating the pixel values into at-satellite reflectance. |
Post-processing of images: |
| Two interaction goals followed in this study. In first stage, remote sensing techniques used in evaluation of surface changes, determination the type of land cover/use classes. In next stage, area evaluated for environmental vulnerability by using prominent land degradation indicators method and GIS tools and then to analyses the impacts of land use/cover class expansion on environmental vulnerability. |
Environmental vulnerability indicators selection and weights of the indicators: |
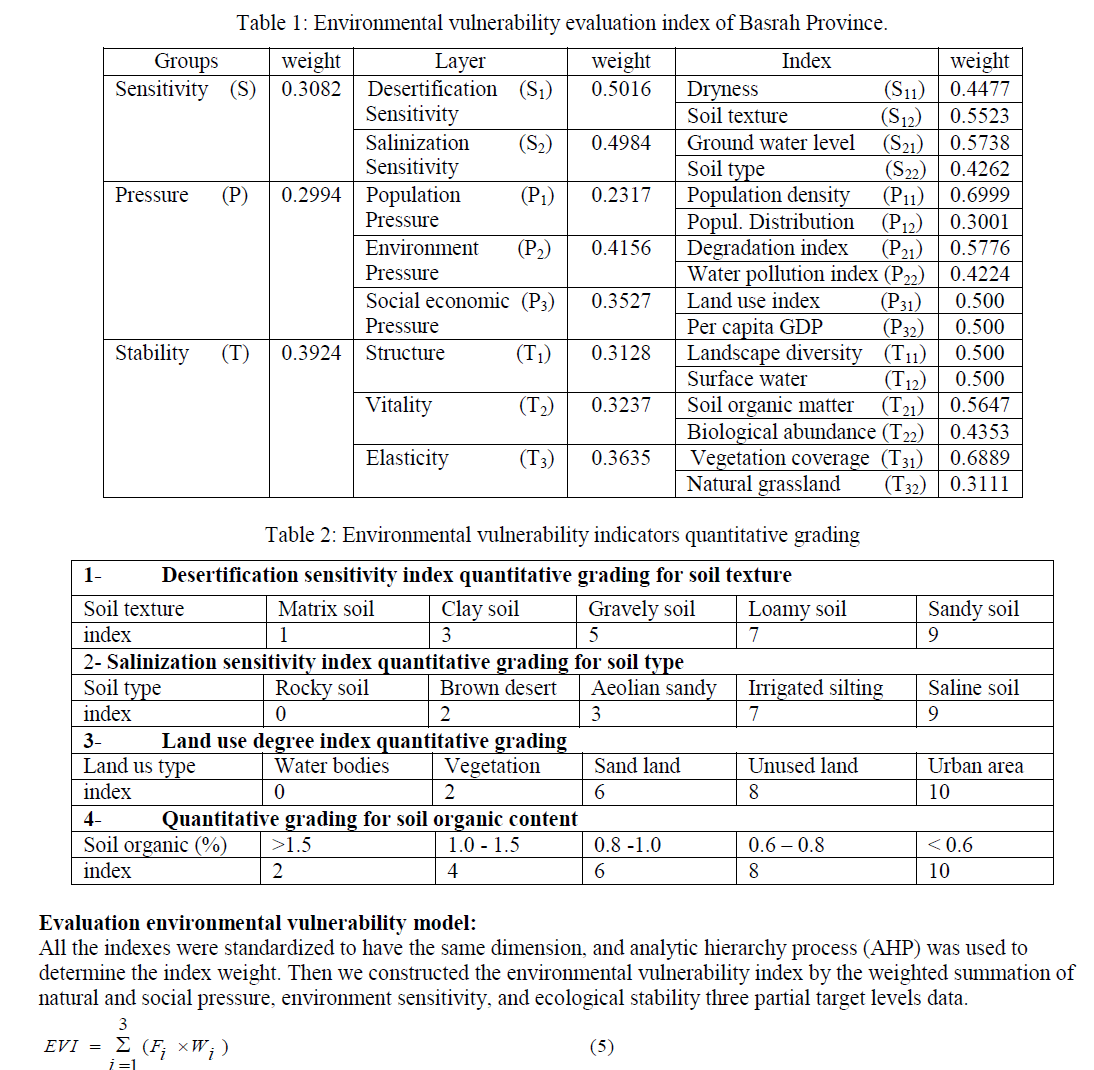
| Environmental vulnerability indicators were selected using the approach suggested by Alewell and Manderscheid [21- 22]. According to the ecological vulnerability definition and regional main environment problem, the evaluation indicator system was established by three groups including natural and social pressure, environment sensitivity, and ecological stability, and 16 factors were selected. The following table (Table 1) shows the three levels of environmental vulnerability evaluation index for Basrah Province, Iraq. The contribution of each indicator for ecological vulnerability is usually different, which can be indicated by a weight value. The analytical hierarchy process (AHP) was used to determine the weights of each factor in this study [23-24]. |
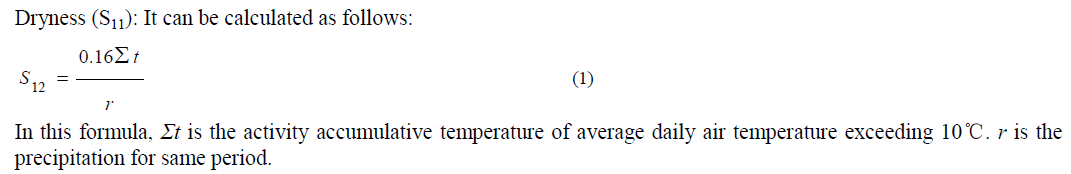 |
| Soil texture (S12): It can reflect the ability of soil erosion resistance and the desertification will be easily happening along with soil particle increasing. Combing several experts’ advice, we get the standardized score of desertification sensitivity for different soil texture (Table2). |
| Ground water level (S21): We collected ground water level from 28 existing wells. Then import these data into ArcGIS software based on their geographical coordinates. Based on Kring’s interpolation algorithm, we made ground water level map. |
| Soil types (S22): We assigned the standardized score to different soil types by consulting experts in the fields concerned, and the result is as follows (Table2): |
| Population density (P11) and Distribution (P12): Considering large part in arid region is desert, it is not comparable to represent the population density purely through dividing regional population by area. The desert area should be deducted. According to the classification system, desertification area is removed from the total area of evaluation unit. |
| Degradation index (P21): Degradation is the direct response of environment to human activities. It is the ratio of degradation area to total area in evaluation unit and Land salinization index: It is the ratio of salinization area to total area in evaluation unit. |
| Water pollution index (P22): PH value, salt content, and SO4 content exceeding rate are collected to establish water pollution index as follows: |
 |
 |
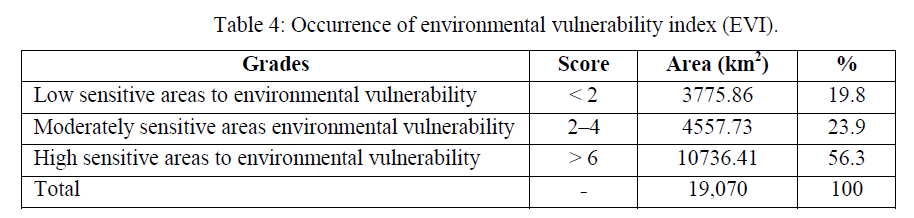
| In this formula, EVI is environmental vulnerability index, Fi represents for standard value of natural and social pressure, environment sensitivity, and ecological stability. ïü÷i is the weight of three indices. The result was computed from EVI model is a continuous value, which should be classified into several levels standing for different environmental vulnerability. According to the existing research results, combined with the natural conditions of Basrah Province, we divided vulnerability into three grades. Each grade has a corresponding environmental vulnerability index (EVI). The results are listed in Table 4. |
RESULTS AND DISCUSSION |
Environment sensitivity index assessment: |
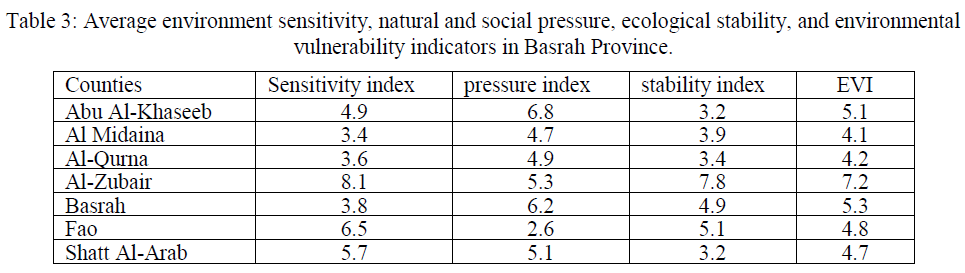
| The term ‘environment sensitivity’ includes the desertification sensitivity index and the salinization sensitivity index, both of which are considered to be key components of terrestrial ecosystems since they are vital to land protection, biodiversity, hydrological and geochemical cycles, climate and many other facets [27]. According to the formula (5), the value of the minimum environmental sensitivity index of the study area is at Al Midaina, with an index of 3.4, and the maximum is at Al-Zubair with an index of 8.1. The average environmental sensitivity index is 5.2 (Table 3). The proportion of the light sensitive area is 21.9%, moderate sensitive area proportion is 26.5% and severe sensitive area proportion reaches to 51.8%. The sensitivity level in Basrah Province obviously presents the characteristics of increasing from north to south. The sensitivity index of Al Midaina and Al-Qurna in the northern Basrah Province are almost among 3.4 to 3.6, and the other counties are between 3.8 and 8.1, which have reached serious sensitivity level. The main reason for high sensitivity in these counties is due to the sand drifting and higher ground water level and stronger evaporation, which cause serious salinization. |
Natural and social pressure index assessment: |
| A significant increase in Basrah Province’s urbanized areas between 1990 and 2003 was observed, with the annual rate of expansion in each county varying from 0.8% to 2.1%. As southern Iraq is considered a national economic and administrative centre, the percentage increase in its total urban area of 20.6% during the 10-year study period is perhaps unsurprising [28]. The value of natural and social pressure degree of whole study area and each county can be worked out, shown in Tables 3. The minimum pressure degree index is 2.6, the maximum is 6.8, and the average pressure degree index is 5.1. Average pressure index results (Table3) of every county show that average pressure index of Abu Al-Khaseeb, Al-Zubair, Basrah, and Shatt Al-Arab are all above 5. Especially for Abu Al-Khaseeb, the average pressure index has reached to 6.8, and 39.7% of area is serious pressure region, which has a serious threat to regional ecological security. Among 7 counties, Al Midaina, Al-Qurna, Fao have the minimum average pressure degree index, and the reason is that these three counties located in Basrah Province, which has low land use degree index. |
Ecological stability index assessment: |
| Ecological conversion process occurring in Basrah Province can be characterized in terms of four strands: (1) Degradation of vegetation cover accounted for more than 2.7 % (over 514.8 km2) and wetland loss more than 0.9 % of the total land area (over 171.6 km2); (2) Desertification affected more than 2.6 % of the total land area (over 495.8 km2); (3) More than 0.5 % was affected by urbanization (loss of agricultural land use) (over 95.4 km2) and (4) Some 1.3 % of the study area was converted into salinized land (more than 247.9 km2) between 1990 and 2003 [28]. The stability index in Basrah Province is between 3.1 and 7.8, and average stability index is 4.6, which belongs to unstable level. Stability of west is obviously higher than that in eastern region. Comprehensive stability is poorer in Al-Zubair County in the western Basrah Province with the average stability index of above 7. This is because most of the area in these counties are desert, the soil organic content is just 0.2, and the diversity index is only one third of other region. But five counties in southeast such as Abu Al-Khaseeb and Shatt Al-Arab have relative high vegetation coverage and landscape diversity, the average stability index is about 3.2. In general, most area of the Basrah Province is in an unstable state and it is important to enhance the construction of ecological environment restoration. |
 |
Distribution land quality index: |
| The spatial distribution map of environmental vulnerability was prepared by using ARC/GIS software after the completion environmental sensitivity index, natural and social pressure index and stability index according to their corresponding weights and got the environmental vulnerability index. Table 4 showed that the study area could generally be divided into three regions and the characteristics of land degradation in Basrah Province are concluded as follows: (1) the maximum environmental vulnerability index is 7.2 in Al-Zubair County, and the minimum value is 4.1 in Al Midaina. The average environmental vulnerability index of the whole region is 5.1, which lies in a moderate vulnerability level. Moderate environmental vulnerability region accounts for 23.9% of the total area, light environmental vulnerability region and serious vulnerability region account for 18.8% and 56.3% respectively. More than 75% of the area has reached serious environmental vulnerability level, and nearly one-fifth of the area belongs to moderate vulnerability level. (2) The spatial distribution characteristics of environmental vulnerability are demonstrated in two aspects: one is that the vulnerability degree is obviously increasing from east to west; The other is that environmental vulnerability degree in inner Basrah Province is significantly lower than that in the outer Basrah Province. Scarce vegetation, intense evapotranspiration, strong winds and erosion in the outer of Basrah Province all lead to serious ecological environmental vulnerability. |
 |
Analysis of land degradation driving forces: |
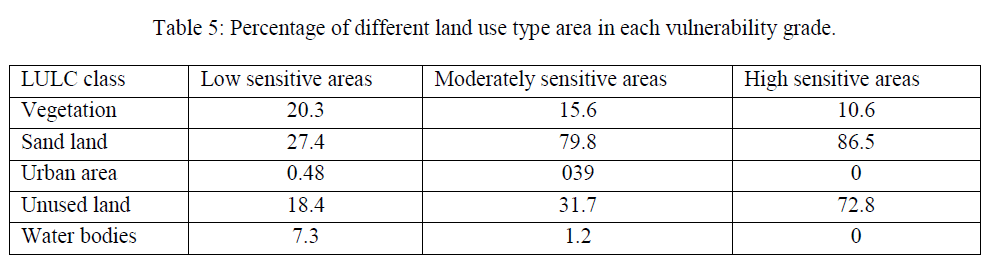
| Environmental vulnerability is a complex process. In this research, an environmental vulnerability model for detecting the vegetation and sand drifting change of soil surface was applied so as to monitor the environmental vulnerability process. This study has looked into the possibility of applying data collected by the land use variation survey to study the anticipated relationship between land uses/ cover changes and land degradation in the south part of Iraq. The west of Basrah Province belongs to serious water lack region, due to the shortage of water resources, causing a series of environmental problems, such as water table declining, drying up, vegetation degradation, land desertification and so on. With the interference of human activities, environment has become more and more vulnerable. In one sense, we may say that the agricultural land quality of Basrah Province is the result of primary degradation from climatic and geomorphic conditions, and secondary degradation from human interference. Land use types can reflect human activity intensity, in order to reveal the influence of human activities on vulnerability like overgrazing and deforestation; we analyzed the change of different land use type area in each environmental vulnerability grade. The results show that environmental vulnerability has a close relationship with land use type in study area (Table 5). The proportion of desertification land area increases from 21.6% to 56.3% along with vulnerability enhancing [28]. Desertification land, which has great interfering with human, has significantly positive correlation (r= 87.9) with degradation degree. Along with population growth and rapid economic development of Basrah Province, large scale development of resources would exacerbate desertification, and the existing oasis is also under the threat of imbalance of the environment and ecosystem. Water resources have become the important constraint factor for economic development, therefore, we should put more effort on the management of water resources to ensure the safety of ecologic water, and to reduce environmental problem have great significance for obtaining the best social, ecological, economic benefits and the regional sustainable development. |
 |
CONCLUSION |
| Evaluation of environmental vulnerability on a regional scale is important because vulnerability is incompatible with sustainable development. Policy makers involved in land use planning require tools to evaluate environmental vulnerability so they can go on to develop measures aimed at protecting and conserving environment. Numerous studies had been carried out in assessing environmental vulnerability aiding with various mathematical methods for sampling strategy, indicator selection, weight determination and model construction. With the advance of spatial information science, geo-information technologies provide powerful tools for data manipulation and information acquisition, which may, along with the previously stated methods, bring the research of land quality assessment into a new era. This study concluded that (1) from the meaning and the cause of environmental vulnerability, we built multiobjective and multilayer land quality assessment index system framework based on natural and social pressure index, environment sensitivity index, and ecological stability index were calculated with the aid of geo-information techniques. A Pressure-State-Respond model and the analytic hierarchy process method were used to evaluate the land quality index. This study indicated that the method that integrates the technologies, such as geo-information techniques, and the AHP approach to evaluate land quality index, can achieve a distributed result and truly reflect the distribution characteristics. (2) The results revealed that land use changes have affected the wider environment and accelerated land degradation, with severe damage located in south western Basrah Province representing 28.1% of the total area. Areas of high to moderate degradation characterize the rest of the south, representing 52.7% of the total area, while the north of the study region is characterized by very low and low degradation levels accounting for 8.5 and 10.7%, respectively. (3) The environmental vulnerability of Basrah Province is the result of primary vulnerability from climatic and geomorphic conditions, and secondary vulnerability from human interference. Now desertification is the most serious environmental problem in this study area, and the land degradation has great relation with desertification. With the increase of vulnerability level, the proportion of desertification area is greatly improving. The key of ecological restoration and reconstruction is to comprehensively harness desertification and ensure ecological water use to the greatest extent. (4) Iraq faces serious environmental vulnerability problems that must be addressed immediately; failure to do so will greatly compound the cost and complexity of later remedial efforts, with environmental degradation beginning even now to pose a major threat to human well-being, especially among the poor. |
ACKNOWLEDGMENTS |
| This research was supported by the Basrah University, Iraq and the Huazhong University of Science and Technology, the Project of Science and Technology of Wuhan City of China under two Grants No. 20 0150 05037 and No. 20 0550 03059-34 and the Natural Science Foundation of Hubei Province under Grant No. 20 05ABA0 47. |
References |
Keywords |
| Chalcones, o-hydroxyacetophenone, pyrazolines, antibacterial, antifungal agents. |
INTRODUCTION |
| Ultrasound has increasingly been used in organic synthesis[1,2]. This method is more convenient as it requires shorter time for completion of reaction and higher yields are obtained as compare to conventional methods. |
| Pyrazolines and their derivatives have been found to possess a broad spectrum of biological activities such as anti-bacterial[3-5], anti-depressant[6], anti-convulsant[7-9], anti-hypertensive[10], anti-oxidant[11], anti-tumor[12] and anticancer activities[13,14]. Recently these classes of compounds are reported to possess potential anti-viral activity against flavivirus[15], HIV[16] and anti-malarial activity[17]. |
| Pyrazolines are well known and nitrogen-containing five membered heterocyclic compounds. Several pyrazoline derivatives have been found to possess wide range of biological activities[18]. Benzofuran bearing 1,3,5- trisubstituted pyrazoline exhibited antitubercular, antimicrobial and anti-inflammatory activities[19]. Pyrazoline derivatives[20] have been associated with various bioactivities hence various methods have been worked out for its synthesis. The discovery of the analgesic and antipyretic properties of antipyrine, phenylbutanolinone, the considerable biological activity of fused pyrazoline and the excellent dying properties of Pyrazolyl azo derivatives (eg restazine) have undoubtedly promted interest in development of new derivatives. |
| Literature survey indicated that the only few pyrazoline are naturally occurring. This is due to the difficulty of living organisms to build an N-N bond pyrazoline itself and many N unsubstituted derivatives are inhibitors and deactivators of liver alcohol dehydrogenage. The use of pyrazoline derivatives in medicine are undoubtedly the principle application. Some of the most important aromatic pyrazolines with biological properties like amebicide,vrichomonacidal, hypoglycaemic, antipsychotic, sedative, hypnotic were reported. Pyrazolines of sulphonamides possess wide range of bacteriostatic and fugicidal actions. For examples bacteriostastic action in vivo for a prolonged time was noted in orisul. Certain alkyl pyrazolines were also found to have the above properties. A sharply pronounced sedative action on the central nervous system was shown by alkyl aryl pyrazolines. Stanazolol is used as an analbolic steroid with no adverse side effects. |
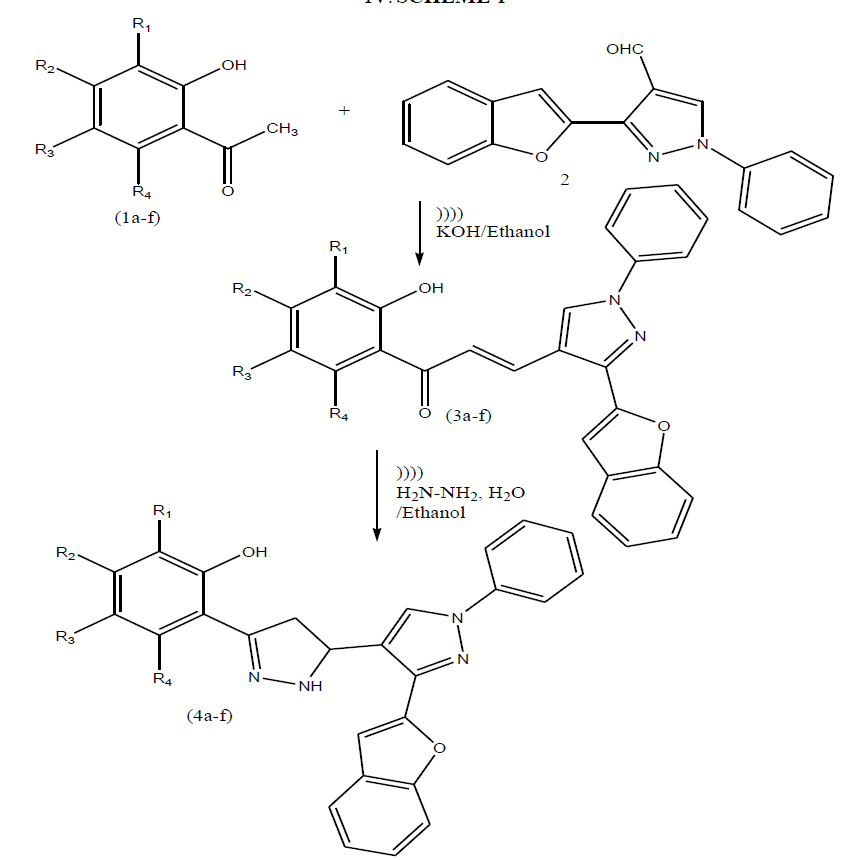
| Keeping in view of these observations and in continuation of our work on chalcones and pyrazoline derivatives herein we wish to report synthesis of these heterocycles (scheme-I) by using ultrasound method containing benzofuran moiety. |
MATERIALS AND DISCUSSION |
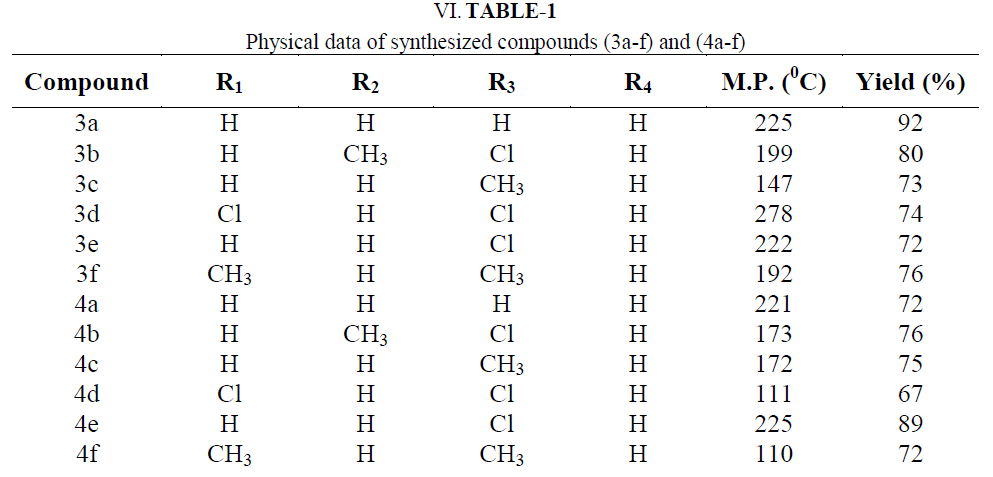
| For this invention chemicals with s.d fine and Aldrich make were used from local dealer. 99.99 % pure chemicals, thin layer chromatography and melting point, mixed melting point had been used to check the purity of the chemicals. O- hydroxy acetophenones as a precursor synthesized by conventional method. Chalcones (3a-f) and pyrazolines (4a-f) were synthesized by non conventional method. |
RESULTS AND DISCUSSION |
| All experiments under ultrasonication were carried out in bath type ultrasonicator model EN-20U-S manufactured by Enertech Electronica Pvt. Ltd. Mumbai, India having maximum power out put of 100w and 33KHz frequency. All the newly synthesized compounds were screened for its in vitro antibacterial activity against Pseudomonus aerugninasa, Straphylococcus aureus and E. coli using Gentamicin and tetracycline as a reference standard by paper disc diffusion method. Antifungal activity was evaluated against Candida sp. Using Ketoconazole as a standard drug. All the tested compounds were evaluated at 100 ug/ml conc. Some of the compound has shown moderate antimicrobial activities. |
SCHEME-I |
 |
EXPERIMENTAL SECTION |
| All the recorded melting points were determined in open capillaries in liquid paraffin bath and are uncorrected. The progress of reaction was monitored with thin layer chromatography using silica gel-G(Merck). IR spectra have been recorded on a Perkin Elmer Spectrum Version 10.4.2 FTIR Spectrophotometer. 1HNMR spectra have been recorded on Bruker Avance-II 400 MHz NMR spectrophotometer using CDCl3 as a solvent and tetra methyl silane as internal standard. Signal values have been shown in δ (ppm). The Mass spectra have been recorded on a Waters, QTOF Micromass (LC-MS) mass spectrometer. The antimicrobial activity of the synthesized compounds have been tested by disc diffusion method. |
Synthesis of 3-(3-benzofuran-2-yl)-1-phenyl-1H-pyrazol-4-yl)-1-(2-hydroxyphenyl)prop-2-en-1-one (3a): |
 |
 |
ANTIMICROBIAL ACTIVITY |
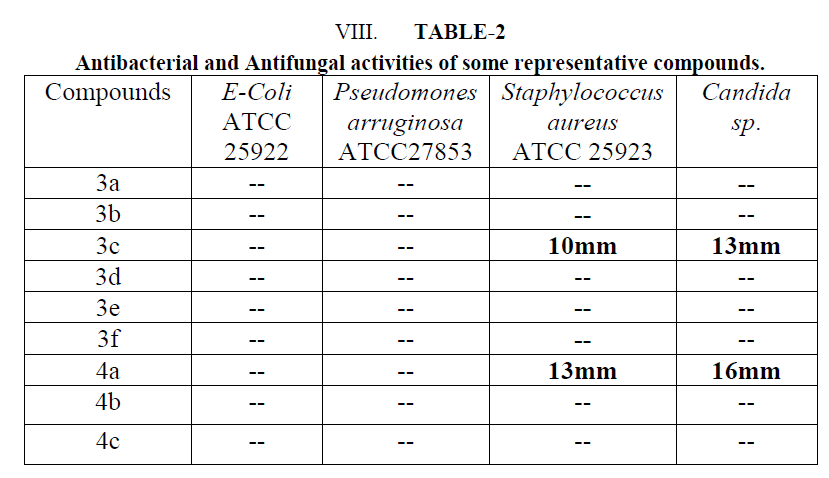
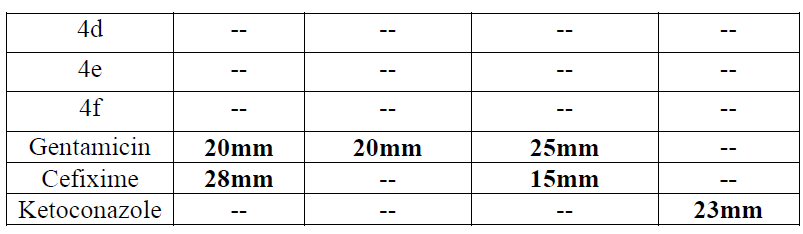
| These synthesized compounds were screened for its in vitro antimicrobial activity against gram positive organism pseudomonas aeruginosa and staphylococcus aureus and gram negative organism E. Coli using Gentamycin and Cefixine as a reference standard by paper disc diffusion method. Antifungal activity was evaluated with Ketaconazole against Candida as a standard. All these tested compounds were evaluated at 100 μg/ml concentration. Muller Hinton agar was used culture media. The zone of inhibition was measured in mm after 24 hr of incubation at 370C. Microbial data for corresponding compounds are summarized in Table-2 |
| An examination of data revealed that 3c and 4b have shown moderate activity for gram positive bacteria Staphylococus aureus ATCC 25923 (10-13mm). Compound 4b was found to be more potent with respect to Cefixime. Similarly, these compounds have shown moderate antifungal activity for Candida sp. (13-16 mm). |
 |
 |
CONCLUSION |
| The newly synthesized pyrazoline derivatives are very easy to carry out giving high yield, shorter time, environmentally benign reactions. These results make interesting lead molecule for further synthetic and biological evaluation. |
ACKNOWLEDGEMENTS |
| The author is thankful to Dr. A. B. Nikumbh The Head, Dept of Chemistry & Principal Dr. K. P. Kakade SSGM College, Kopargaon for providing laboratory facilities and for constant encouragement.The author is thankful to Uday Khedakar, Director, BAC-TEST Laboratory Nashik for antimicrobial analysis and UGC (WRO) for providing financial assistance. Author is also thankful to the Director, SAIF, Panjab University Chandigarh for providing spectral data. |
References |
|