ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
|
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
The chemical equilibria of multinuclear complexation of toxic metal ions Cd (II) and Pb (II) with 2- Amino-3-(4-hydroxyphenyl) propanoic acid and 2, 4-dihydroxypyrimidine ligands in aqueous solution were studied by potentiometric technique. The metal ligand formation constants of investigated binary, ternary and quaternary metal chelates were determined at 3710C and fixed ionic strength I =0.1M NaNO3 by using SCOGS and the complex formations were studied on the basis of computer analysis. Solution structure and stability order of studied binary (MA, MB) ternary (MAB) and quaternary (M1M2AB) complexes have been discussed.
Keywords |
| Formation Constant, SCOGS, Distribution Curves, Complexes, Stability Order. |
I. INTRODUCTION |
| A large number of coordination compounds formed by metals in which the metal atoms are bound to a number of electron donating anions or neutral molecules called ligands. Two or more donor atoms of a ligand bind single metal ion and form a heterocyclic ring structure, it is said to be a chelating ligand and the complex compound itself is termed as metal chelate. The present paper is concerned with the equilibrium study of ternary and quaternary metal chelates of biological significance. In this paper a valuable study have been done on mixed ligand (MAB) ternary systems and (M1M2AB) quaternary system of Cd (II) and Pb (II) with Amino acid as primary ligand (A) and pyrimidine base which are used in many biological, medicinal and agricultural applications as secondary ligand (B). The toxic metal ions inevitably exist as metal complexes in biological systems by interaction with the numerous molecules possessing groupings capable of complexation or chelation. Mixed chelation occurs commonly in biological fluids, i.e. as million of potential ligands are likely to compete for metal ions found in vivo. It is an important phenomenon[1] in the coordination chemistry of living organisms. |
| 2-Amino-3-(4-hydroxyphenyl) propanoic acid is one of the 22 amino acids that are used by cells to synthesize proteins and is a non-essential amino acid with a polar side group. It is a precursor to neurotransmitters and increases plasma neurotransmitter levels. A number of studies have found that it would be useful during conditions of stress, cold, fatigue [2] loss of a loved one such as in death or divorce, prolonged work and sleep deprivation[3]. The 2, 4- dihydroxypyrimidine is a pyrimidine base which is common and naturally occurring .It was isolated the hydrolysis of yeast nuclein [4] that was found in bovine thymus and spleen, herring sperm, and wheat germ[5]. It is a planar, unsaturated compound that has the ability to absorb light [6]. It is the hydrogen bond acceptor and can form two hydrogen bonds. It can also bind with a ribose sugar to form a ribonucleoside, uridine. When a phosphate attaches to uridine, uridine 5'-monophosphate is produced [7] |
II. RELATED WORK |
| T. R. Barman and G. N. Mukherjee [8] studied mixed ligand complex formation equilibria of Co (II), Ni (II), Cu (II) and Zn (II) with glycine and 4, 6- dimethyl -2- guanidinopyrimidine. Shaesta Quyoom and Badar-Uddin-Khan [9] described potentiometric and U V spectral studied of binary and ternary complexes of some metal ions with N –acetylcystine and amino acids. Patel et al [10] have determined proton ligand constants for peptides glyclalanine and glycylvaline and imidazoles (imidazole, 2-methyl imidazole and 2-ethyl imidazole), hydrolytic constants for three metal ions Cu (II), Ni (II) and Zn (II) by potentiometric method. H.N.Aliyu and J Na’ aliya [11] gave potentiometric studies on essential metal (II) amino acid complexes. |
III. MATERIAL AND METHOD |
| In the study of formation of complexes in solution, two kinds of stability of complexes can be seen. The thermodynamic stability deals with metal – ligand bond energies, stability constants etc. and the kinetic stability refers to the speed with which transformation leading to the attainment of equilibrium will occur. The kinetic stability deals with the rate and mechanism of chemical reactions as well as with the thermodynamic variables involved in the formation of intermediate species or activated complexes. |
| The complex formation in solution proceed by the stepwise addition of the ligands to the metal ion, a number of successive equilibria can be formulated: |
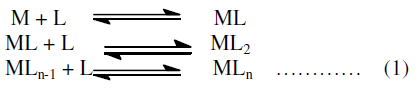 |
| Equation (1) can be given according to the Law of Mass Action as under: |
 |
| In the above equations “n” represents the coordination number of the metal ions, terms in bracket [ ] refers to the activities of different species and Kn is thermodynamic stepwise stability constant or formation constant. |
 |
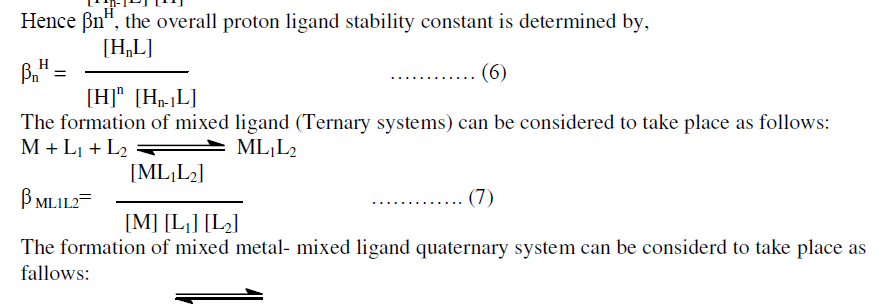
| βn = overall stability constant which is the product of stepwise formation constant. |
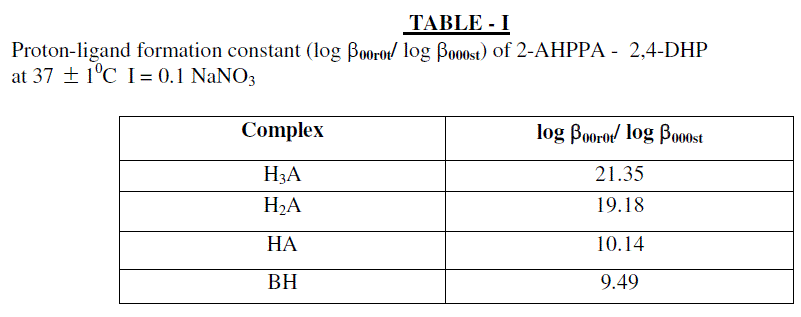
| The protonation of the ligand L occurs in steps exactly in the same way as complexation reaction consequently following proton ligand equilibria may be considered. |
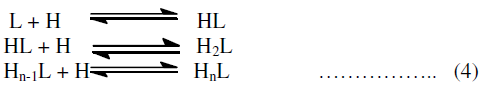 |
| Finaly the proton ligand stability constants thus expressed as: |
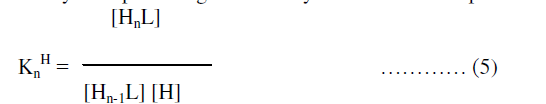 |
 |
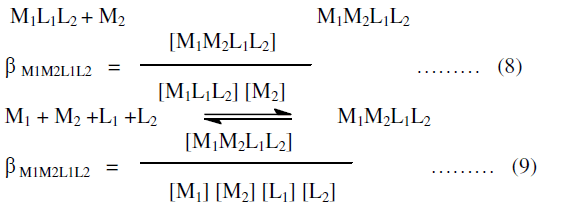 |
| pH- METRIC TITRATION |
| The pH measurements were done by Bjerrum's [12] method modified by Irving and Rossotti [13] with \ an electric digital pH meter (Eutech 501) with a glass electrode supplied with the instrument and working on 220V/50 cycles stabilized by A.C. mains. The pH meter has a reproducibility of ±0.01 pH. Potentiometric titrations of each ligand with standard carbonate free sodium hydroxide were carried out at 3710C and I = 0.1M NaNO3. The pH meter was calibrated before starting observation with buffer solutions of pH (4.0) and pH (9.2) respectively which were prepared by dissolving buffer tablets (BDH) in double distilled water in appropriate concentrations. After completion of whole set, the calibration was again checked with buffer of pH = 4.0 and was found to remain unaltered. All the metal salts used were of A.R. grade and were standardized volumetrically by titration with the disodium salt of EDTA is presence of suitable indicators, as described by Schwarzenbatch [14]. The entire chemicals used were also A.R. grade and were used without further purification. |
| Various solutions were prepared by keeping the total volume 50 ml in following manner: |
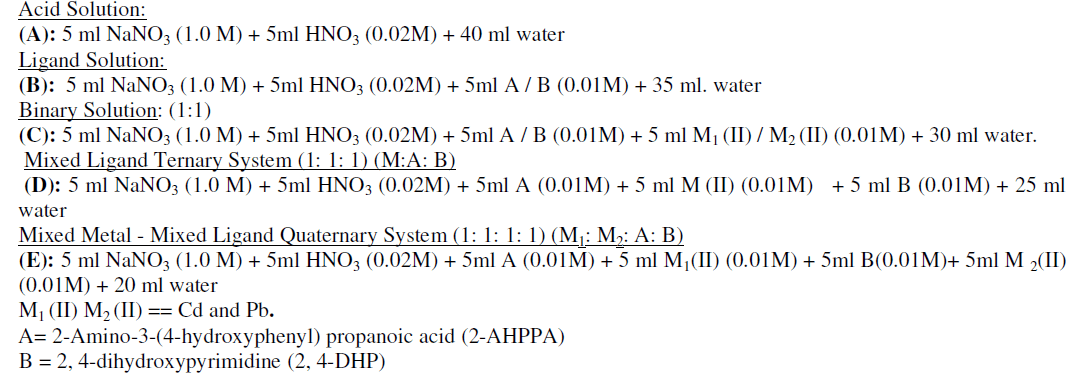 |
| Individually the above solutions were titrated against alkali (NaOH). The pH meter reading with progressive addition of alkali to the titration mixtures were noted, when the reading of pH meter stabilized. The entire experimental work were proceed in an atmosphere of purified nitrogen by bubbling it thrown the solution in which the electrode was dipping. The nitrogen gas thus served to prevent atmospheric oxidation and also to stir the solution. The ionic strength of all mixture solutions |
| was kept 0.1M NaNO3. The free acid concentration was kept equal in each case i.e. 0.02M,strength of the ligand was 0.001M, strength of M (II) was 0.001 M and strength of alkali was kept 0.1 M |
IV. RESULTS AND DISCUSSION |
| Sayce [15] developed a new computer program SCOGS (Stability constant of generalized species) which employs the conventional non linear least square approach. The program is written in FORTRAN IV. It is capable of calculating simultaneously or individually , association constants for any of the species formed in the system containing up to two metals and two ligands, provided that the degree of complex formation is pH-dependent. Thus, SCOGS may be utilized to analyse appropriate pH titration data to yield metal-ion hydrolytic constants, stability constants of simple complexes (MA, MB and MA2 etc.). SCOGS may also be used to calculate constants for "mixed" complexes containing two different metals and two different ligands. |
 |
| In above equation the stoichiometric numbers p, q, r and s are either the zero or positive integer and t is a negative integer for a protonated species, positive integer for a hydroxo or a deprotonated species and zero for a neutral species. |
| TITRATION AND SPECIES DISTRIBUTION CURVES |
| The pH titration curves were obtained by plotting pH vs. volume of alkali and finaly sketched by running the computer program ORIGIN 4.0 which are given in fig. 1 |
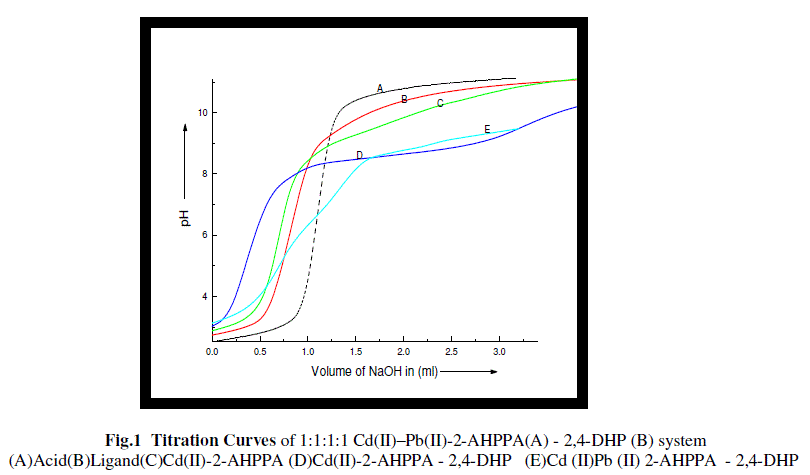 |
| In the curves, “A” represents the Acid, “B” represent the Ligand “C” represent the binary complex, “D” represents the mixed ligand ternary complex and “E” represents the mixed metal - mixed ligand quaternary complex. |
| The species distribution curves were obtained by plotting percent (%) concentration of the species obtained through SCOGS computer programme against pH which are also finally sketched by ORIGIN 4.0 given in fig. 2 |
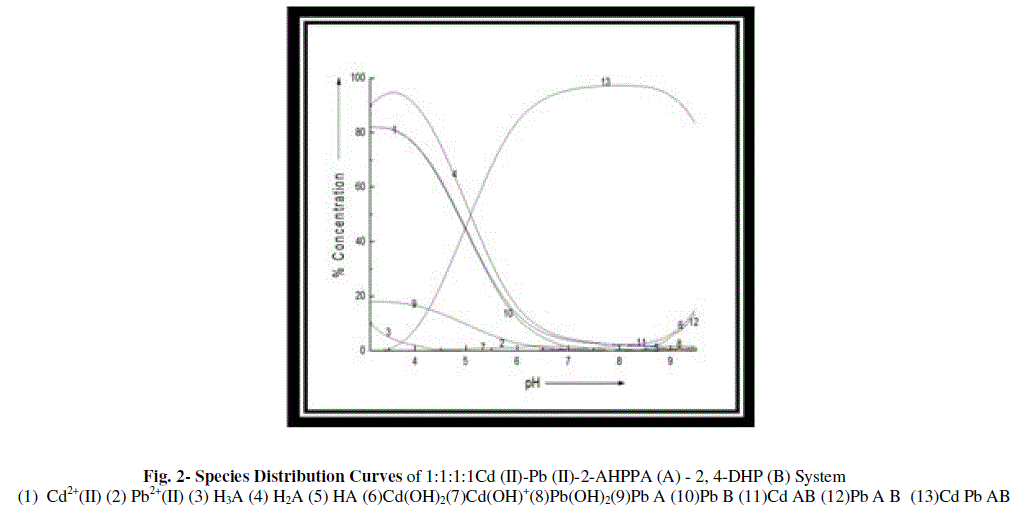 |
| The binary complex Pb- A exist maximum concentration ~18% at the start of titration while Pb B complex attain the maximum concentration ~82% at the start of titration shows declining trend with increase in pH range. Ternary complexes Cd A B existed with lower amount while the Pb A B existed with maximum concentration ~13.8% at the ~ 9.5 pH. The major species which is quaternary complex Cd Pb AB attain the maximum concentration ~97.% at the ~ 8.2 pH. Due to ligand - ligand interactions extra stabilization caused and stable metal chelates were formed. |
CHEMICAL EQUILIBRIA OF COMPLEX FORMATION |
| The binary, ternary, quaternary complex and general hydrolytic equilibria have been derived on the basis of distribution curves of the complexes (obtained through SCOGS) occurring at different pH. Following equilibria have been proposed for all the above systems respectively. |
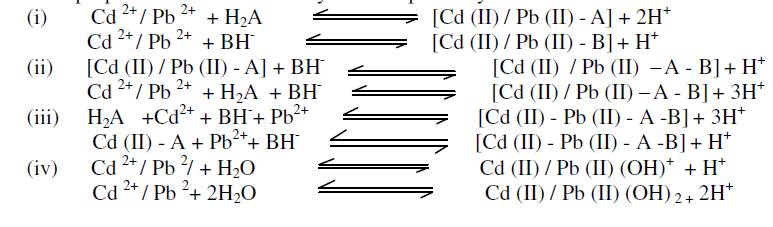 |
 |
SOLUTION STRUCTURE OF INVESTIGATED COMPLEXES |
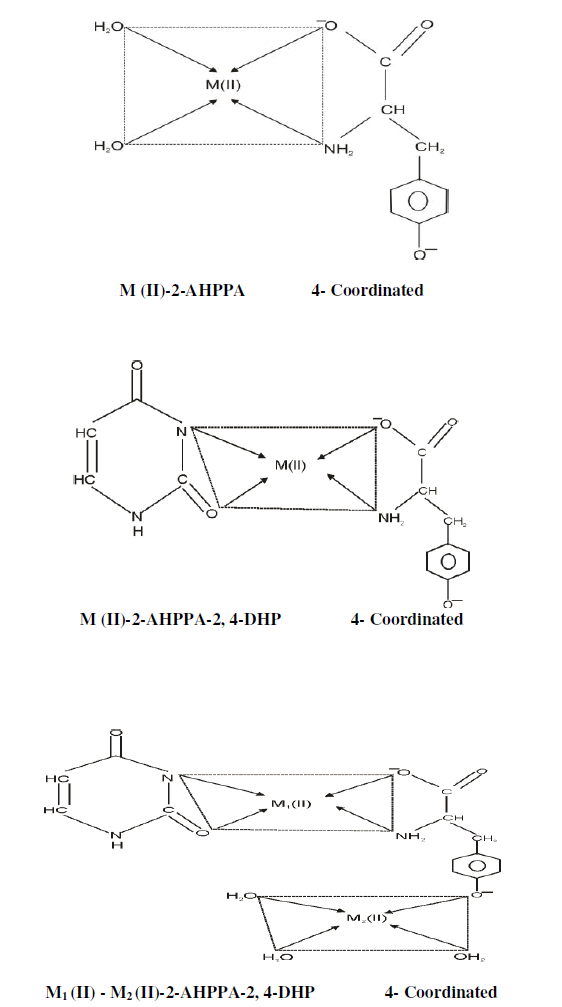 |
V. CONCLUSIONS |
| Metal toxicity is a major problem for human and other living beings. Toxicity is a function of solubility. Insoluble compounds as well as the metallic forms often exhibit negligible toxicity. Toxic metals may be made insoluble or collected, possibly by the aid of chelating agents. The chelating agents are substances that have a strong ability to grab onto toxic metals and dislodge them from the tissues so they can be removed. The ligands used in this work are very good chelating agents which interacted with metal ions to form various metal chelates and remove the toxicity of metals.So this work provides a very good way for study to remove the toxicity of metals. |
| OVERALL STABILITY ORDER OF INVESTIGATED COMPLEX SPECIES: |
| Cd--Pb (II)-2-AHPPA (A) - 2, 4-DHP (B) > Pb (II)-2-AHPPA (A) - 2, 4-DHP (B) > |
| Cd (II)-2-AHPPA (A) - 2, 4-DHP (B) > Pb (II) -2, 4-DHP (B) > Cd (II) - 2, 4-DHP |
| (B) > Pb (II)-2-AHPPA (A) > Cd (II)-2-AHPPA (A) |
References |
|